Abstract
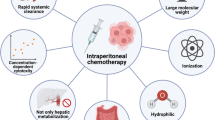
Peritoneal carcinomatosis remains a significant cause of morbidity and is a marker of poor prognosis in a range of malignancies, including those of the gastrointestinal and gynaecological tracts. In these cases, regional therapy has been explored as a treatment strategy to take advantage of the prolonged confinement of such tumours within the peritoneal cavity and the steep dose-response relationship for most cytotoxic agents. The pharmacokinetic rationale is based on exploiting the peritoneal-plasma barrier, which slows the rate of drug clearance from the peritoneal to systemic compartments and creates a concentration differential in favour of the peritoneal cavity. This allows higher drug concentrations, and thus increased cytotoxicity, to be achieved at the site of a tumour within the peritoneal cavity. There is pharmacodynamic evidence from a number of clinical trials to support the translation of these pharmacokinetic advantages of intraperitoneal chemotherapy into clinical benefit. However, its clinical application remains controversial because of concerns regarding intraperitoneal drug distribution, technical challenges and toxicity associated with regional drug delivery and the clinical relevance of the studies undertaken. The purpose of this review is to summarize the pharmacokinetic rationale of intraperitoneal drug delivery, review the pharmacodynamic studies performed in ovarian, colorectal and gastric cancers, and outline the future directions and challenges in the clinical application of this mode of treatment.












Similar content being viewed by others
References
Warrick C. An improvement on the practice of tapping; whereby that operation, instead of a relief for symptoms, becomes an absolute cure for an ascites, exemplified in the case of Jane Roman; and recommended to the consideration of the Royal Society, by Christopher Warrick, of Truro, Surgeon. Philos Trans R Soc Lond B Biol Sci 1744; 43: 12–9
Fujiwara K, Armstrong D, Morgan M, et al. Principles and practice of intraperitoneal chemotherapy for ovarian cancer. Int J Gynaecol Cancer 2007; 17: 1–20
Weisberger AS, Levine B, Storaasli JP. Use of nitrogen mustard in treatment of serous effusions of neoplastic origin. JAMA 1955; 159: 1704–7
Dedrick RL, Myers CE, Bungay PM, et al. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep 1978; 62: 1–11
Howell SB. Pharmacologic principles of intraperitoneal chemotherapy for the treatment of ovarian cancer. Int J Gynecol Cancer 2008; 18 Suppl. 1:20–5
Balthasar JP, Fung HL. Pharmacokinetic and pharmacodynamic optimization of intraperitoneal chemotherapy. Life Sciences 1996; 58(7): 535–43
Markman M. Intraperitoneal chemotherapy. Semin Oncol 1991; 18: 248–54
Speyer JL, Collins JM, Dedrick RL, et al. Phase I and pharmacological studies of 5-fluorouracil administered intraperitoneally. Cancer Res 1980; 40: 567–72
DeGregorio MW, Lum BL, Holleran WM, et al. Preliminary observations of intraperitoneal carboplatin pharmacokinetics during a phase I study of the Northern California Oncology Group. Cancer Chemother Pharmacol 1986; 18: 235–8
Zimm S, Cleary SM, Lucas WE, et al. Phase I/pharmacokinetic study of intraperitoneal cisplatin and etoposide. Cancer Res 1987; 47: 1712–6
Alberts DS, Surwit EA, Peng YM, et al. Phase I clinical and pharmacokinetic study of mitoxantrone given to patients by intraperitoneal administration. Cancer Res 1988; 48: 5874–7
Blochl-Daum B, Eichler HG, Rainer H, et al. Escalating dose regimen of intraperitoneal mitoxantrone: phase I study 2013 — clinical and pharmacokinetic evaluation. Eur J Cancer Clin Oncol 1988; 24: 1133–8
Kirmani S, McVey L, Loo D, et al. A phase I clinical trial of intraperitoneal thiotepa for refractory ovarian cancer. Gynecol Oncol 1990; 36: 331–4
Malmstrom H, Larsson D, Simonsen E. Phase I study of intraperitoneal carboplatin as adjuvant therapy in early ovarian cancer. Gynecol Oncol 1990; 39: 289–94
Muggia FM, Chan KK, Russell C, et al. Phase I and pharmacologic evaluation of intraperitoneal 5-fluoro-29-deoxyuridine. Cancer Chemother Pharmacol 1991; 28: 241–50
Isonishi S, Kirmani S, Kim S, et al. Phase I and pharmacokinetic trial of intraperitoneal etoposide in combination with the multidrug-resistance-modulating agent dipyridamole. J Natl Cancer Inst 1991; 83(9): 621–6
Markman M, Rowinsky E, Hakes T, et al. Phase I trial of intraperitoneal taxol: a Gynecoloic Oncology Group study. J Clin Oncol 1992; 10: 1485–91
McClay EF, Goel R, Andrews P, et al. A phase I and pharmacokinetic study of intraperitoneal carboplatin and etoposide. Br J Cancer 1993; 68: 783–8
Oza AM, ten Bokkel Huinink W, Dubbelman R, et al. Phase I/II study of intraperitoneal mitoxantrone in refractory ovarian cancer. Ann Oncol 1994; 5: 343–7
McClay EF, Braly PD, Kirmani S, et al. A phase I trial of intraperitoneal carboplatin and etoposide with granulocyte macrophage colony stimulating factor support in patients with intraabdominal malignancies. Cancer 1994; 74: 664–9
Francis P, Rowinsky E, Schneider J, et al. Phase I feasibility and pharmacologic study of weekly intraperitoneal paclitaxel: a Gynecologic Oncology Group pilot study. J Clin Oncol 1995; 13: 2961–7
Plaxe SC, Christen RD, O’Quigley J, et al. Phase I and pharmacokinetic study of intraperitoneal topotecan. Invest New Drugs 1998; 16: 147–53
Hofstra LS, Bos AM, de Vries EG, et al. A phase I and pharmacokinetic study of intraperitoneal topotecan. Br J Cancer 2001; 85: 1627–33
Morgan Jr RJ, Doroshow JH, Synold T, et al. Phase I trial of intraperitoneal docetaxel in the treatment of advanced malignancies primarily confined to the peritoneal cavity: dose-limiting toxicity and pharmacokinetics. Clin Cancer Res 2003; 9: 5896–901
Sabbatini P, Aghajanian C, Leitao M, et al. Intraperitoneal cisplatin with intraperitoneal gemcitabine in patients with epithelial ovarian cancer: results of a phase I/II trial. Clin Cancer Res 2004; 10(9): 2962–7
Oman M, Lundqvist S, Gustavsson B, et al. Phase I/II trial of intraperitoneal 5-fluorouracil with and without intravenous vasopressin in non-resectable pancreas cancer. Cancer Chemother Pharmacol 2005; 56(6): 603–9
Bos AM, De Vos FY, de Vries EG, et al. A phase I study of intraperitoneal topotecan in combination with intravenous carboplatin and paclitaxel in advanced ovarian cancer. Eur J Cancer 2005; 41(4): 539–48
Armstrong DK, Fleming GF, Markman M, et al. A phase I trial of intraperitoneal sustained-release paclitaxel microspheres (Paclimer) in recurrent ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol 2006; 103(2): 391–6
Shinohara H, Okamoto S, Nishitai R, et al. A phase I study of intraperitoneal plus intravenous paclitaxel against gastric cancer with peritoneal dissemination. Gan To Kagaku Ryoho 2006; 33(13): 2027–31
Morgan Jr RJ, Synold TW, Xi B, et al. Phase I trial of intraperitoneal gemcitabine in the treatment of advanced malignancies primarily confined to the peritoneal cavity. Clin Cancer Res 2007; 13(4): 1232–7
Fushida S, Fujimura T, Fukushima N, et al. A multi-centric phase I/II study of intraperitoneal docetaxel combined with S-1 for patients with peritoneal dissemination of gastric cancer. Gan To Kagaku Ryoho 2007; 34(12): 1942–5
Ishigami H, Kitayama J, Otani K, et al. Phase I pharmacokinetic study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer. Oncology 2009; 76(5): 311–4
Fujiwara Y, Nishida T, Takiguchi S, et al. Feasibility study of S-1 and intraperitoneal docetaxel combination chemotherapy for gastric cancer with peritoneal dissemination. Anticancer Res 2010; 30(4): 1335–9
Ishigami H, Kitayama J, Kaisaki S, et al. Phase I study of biweekly intravenous paclitaxel plus intraperitoneal cisplatin and paclitaxel for gastric cancer with peritoneal metastasis. Oncology 2010; 79(3-4): 269–72
Choi MK, Ahn BJ, Yim DS, et al. Phase I study of intraperitoneal irinotecan in patients with gastric adenocarcinoma with peritoneal seeding. Cancer Chemother Pharmacol 2011; 67(1): 5–11
Morgan MA, Sill MW, Fujiwara K, et al. A phase I study with an expanded cohort to assess the feasibility of intraperitoneal carboplatin and intravenous paclitaxel in untreated ovarian, fallopian tube, and primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2011; 121(2): 264–8
Kurita N, Shimada M, Iwata T, et al. Intraperitoneal infusion of paclitaxel with S-1 for peritoneal metastasis of advanced gastric cancer: phase I study. J Med Invest 2011; 58(1–2): 134–9
Howell SB, Pfeifle CL, Wung WE, et al. Intraperitoneal cisplatin with systemic thiosulfate protection. Ann Intern Med 1982; 97: 845–51
Los G, Mutsaers PH, van der Vijgh WJ, et al. Direct diffusion of cis-diamminedichloroplatinum (II) in intraperitoneal rat tumors after intraperitoneal chemotherapy: a comparison with systemic chemotherapy. Cancer Res 1989; 49: 3380–4
Pretorius RG, Petrilli ES, Kean CK, et al. Comparison of the IV and IP routes of administration of cisplatin in dogs. Cancer Treat Rep 1981; 65: 1055–62
Speyer JL, Beller U, Colombo N, et al. Intraperitoneal carboplatin: favourable results in women with minimal residual ovarian cancer after cisplatin therapy. J Clin Oncol 1990; 8: 1335–41
Elferink F, van der Vijgh WJ, Klein I, et al. Pharmacokinetics of carboplatin after intraperitoneal administration. Cancer Chemother Pharmacol 1988; 21: 57–60
Miyagi Y, Fujiwara K, Kigawa J, et al. Intraperitoneal carboplatin infusion may be a pharmacologically more reasonable route than intravenous administration as a systemic chemotherapy: a comparative pharmacokinetic analysis of platinum using a new mathematical model after intraperitoneal vs. intravenous infusion of carboplatin — a Sankai Gynecology Study Group (SGSG) study. Gynecol Oncol 2005; 99: 591–6
Los G, Verdegaal EM, Mutsaers PH, et al. Penetration of carboplatin and cisplatin into rat peritoneal tumor nodules after IP chemotherapy. Cancer Chemother Pharmacol 1991; 28: 159–65
Jandial DD, Messer K, Farshchi-Heydari S, et al. Tumor platinum concentration following intraperitoneal administration of cisplatin versus carboplatin in an ovarian cancer model. Gynecol Oncol 2009; 115: 362–6
Echarri Gonzalez MJ, Green R, Muggia FM. Intraperitoneal drug delivery for ovarian cancer: why, how, who, what, and when? Oncology 2011; 25: 156–65, 170
Ozols RF, Locker GY, Doroshow JH, et al. Chemotherapy for murine ovarian cancer: a rationale for IP therapy with adriamycin. Cancer Treat Rep 1979; 63(2): 269–73
Ozols RF, Locker GY, Doroshow JH, et al. Pharmacokinetics of adriamycin and tissue penetration in murine ovarian cancer. Cancer Res 1979; 39: 3209–14
Guichard S, Chatelut E, Lochon I, et al. Comparison of the pharmacokinetics and efficacy of irinotecan after administration by the intravenous versus intraperitoneal route in mice. Cancer Chemother Pharmacol 1998; 42(2): 165–70
Pestieau SR, Belliveau JF, Griffin H, et al. Pharmacokinetics of intraperitoneal oxaliplatin: experimental studies. J Surg Oncol 2001; 76(2): 106–14
Pestieau SR, Stuart OA, Sugarbaker PH. Multi-targeted antifolate (MTA): pharmacokinetics of intraperitoneal administration in a rat model. Eur J Surg Oncol 2000; 26(7): 696–700
West GW, Weichselbaum R, Little JB. Limited penetration of methotrexate into human osteosarcoma spheroids as a proposed model for solid tumor resistance to adjuvant chemotherapy. Cancer Res 1980; 40: 3665–8
Nederman T, Carlsson J. Penetration and binding of vinblastine and 5-fluorouracil in cellular spheroids. Cancer Chemother Pharmacol 1984; 13: 131–5
Hamilton CA, Berek JS. Intraperitoneal chemotherapy for ovarian cancer. Curr Opin Oncol 2006; 18: 507–15
Reichman B, Markman M, Hakes T, et al. Intraperitoneal cisplatin and etoposide in the treatment of refractory/recurrent ovarian carcinoma. J Clin Oncol 1989; 7: 1327–32
Howell SB, Kirmani S, Lucas WE, et al. A phase II trial of intraperitoneal cisplatin and etoposide for primary treatment of ovarian epithelial cancer. J Clin Oncol 1990; 8: 137–45
Markman M, George M, Hakes T, et al. Phase II trial of intraperitoneal mitoxantrone in the management of refractory ovarian cancer. J Clin Oncol 1990; 8: 146–50
Kirmani S, Lucas WE, Kim S, et al. A phase II trial of intraperitoneal cisplatin and etoposide as salvage treatment for minimal residual ovarian carcinoma. J Clin Oncol 1991; 9: 649–57
Markman M, Hakes T, Reichman B, et al. Phase II trial of weekly or biweekly intraperitoneal mitoxantrone in epithelial ovarian cancer. J Clin Oncol 1991; 9: 978–82
Markman M, Hakes T, Reichman B, et al. Intraperitoneal cisplatin and cytarabine in the treatment of refractory or recurrent ovarian carcinoma. J Clin Oncol 1991; 9(2): 204–10
Markman M, Reichman B, Hakes T, et al. Phase 2 trial of intraperitoneal carboplatin and etoposide as salvage treatment of advanced epithelial ovarian cancer. Gynecol Oncol 1992; 47(3): 353–7
Malmstrom H, Rasmussen S, Simonsen E. Intraperitoneal high-dose cisplatin and etoposide with systemic thiosulfate protection in second-line treatment of advanced ovarian cancer. Gynecol Oncol 1993; 49(2): 166–71
Guastalla JP, Vermorken JB, Wils JA, et al. Phase II trial for intraperitoneal cisplatin plus intravenous sodium thiosulphate in advanced ovarian carcinoma patients with minimal residual disease after cisplatin-based chemotherapy — a phase II study of the EORTC Gynaecological Cancer Cooperative Group. Eur J Cancer 1994; 30A: 45–9
Guastalla JP, Lhomme C, Kerbrat P, et al. Phase II trial of intraperitoneal carboplatin in ovarian carcinoma patients with macroscopic residual disease at second-look laparotomy: a multicentre study of the French Fédération Nationale des Centres de Lutte Contre le Cancer. Ann Oncol 1994; 5:127–32
Malmstrom H, Simonsen E, Westberg R. A phase II study of intraperitoneal carboplatin as adjuvant treatment in early-stage ovarian cancer patients. Gynecol Oncol 1994; 52: 20–5
Braly PS, Berek JS, Blessing JA, et al. Intraperitoneal administration of cisplatin and 5-fluorouracil in residual ovarian cancer: a phase II Gynecologic Oncology Group trial. Gynecol Oncol 1995; 56: 164–8
de Jong RS, Willemse PH, Boonstra H, et al. Phase II study of intraperitoneal cisplatin plus systemic etoposide as second-line treatment in patients with small volume residual ovarian cancer. Eur J Cancer 1995; 31A: 709–13
McClay EF, Braly PD, Kirmani S, et al. A phase II trial of intraperitoneal high-dose carboplatin and etoposide with granulocyte macrophage-colony stimulating factor support in patients with ovarian carcinoma. Am J Clin Oncol 1995; 18: 23–6
Muggia FM, Liu PY, Alberts DS, et al. Intraperitoneal mitoxantrone or floxuridine: effects on time-to-failure and survival in patients with minimal residual ovarian cancer after second-look laparotomy — a randomized phase II study by the Southwest Oncology Group. Gynecol Oncol 1996; 61: 395–402
Barakat RR, Almadrones L, Venkatraman ES, et al. A phase II trial of intraperitoneal cisplatin and etoposide as consolidation therapy in patients with stage II–IV epithelial ovarian cancer following negative surgical assessment. Gynecol Oncol 1998; 69: 17–22
Feun LG, Blessing JA, Major FJ, et al. A phase II study of intraperitoneal cisplatin and thiotepa in residual ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 1998; 71: 410–5
Markman M, Brady MF, Spirtos NM, et al. Phase II trial of intraperitoneal paclitaxel in carcinoma of the ovary, tube, and peritoneum: a Gynecologic Oncology Group Study. J Clin Oncol 1998; 16: 2620–4
Husain A, Sabbatini P, Spriggs D, et al. Phase II trial of intraperitoneal cisplatin and mitoxantrone in patients with persistent ovarian cancer. Gynecol Oncol 1999; 73: 96–101
Morgan Jr RJ, Braly P, Cecchi G, et al. Phase II trial of intraperitoneal cisplatin with intravenous doxorubicin and cyclophosphamide in previously untreated patients with advanced ovarian cancer — long-term follow-up. Gynecol Oncol 1999; 75: 419–26
Morgan Jr RJ, Braly P, Leong L, et al. Phase II trial of combination intraperitoneal cisplatin and 5-fluorouracil in previously treated patients with advanced ovarian cancer: long-term follow-up. Gynecol Oncol 2000; 77(3): 433–8
Rothenberg ML, Liu PY, Braly PS, et al. Combined intraperitoneal and intravenous chemotherapy for women with optimally debulked ovarian cancer: results from an intergroup phase II trial. J Clin Oncol 2003; 21: 1313–9
Sood AK, Lush R, Geisler JP, et al. Sequential intraperitoneal topotecan and oral etoposide chemotherapy in recurrent platinum resistant ovarian carcinoma: results of a phase II trial. Clin Cancer Res 2004; 10: 6080–5
Tiersten AD, Liu PY, Smith HO, et al. Phase II evaluation of neoadjuvant chemotherapy and debulking followed by intraperitoneal chemotherapy in women with stage III and IV epithelial ovarian, fallopian tube or primary peritoneal cancer: Southwest Oncology Group Study S0009. Gynecol Oncol 2009; 112(3): 444–9
Fujiwara K, Nagao S, Kigawa J, et al. Phase II study of intraperitoneal carboplatin with intravenous paclitaxel in patients with suboptimal residual epithelial ovarian or primary peritoneal cancer: a Sankai Gynecology Cancer Study Group study. Int J Gynecol Cancer 2009; 19(5): 834–7
Smith HO, Moon J, Wilczynski SP, et al. Southwest Oncology Group Trial S9912: intraperitoneal cisplatin and paclitaxel plus intravenous paclitaxel and pegylated liposomal doxorubicin as primary chemotherapy of small-volume residual stage III ovarian cancer. Gynecol Oncol 2009; 114(2): 206–9
Markman M, Reichman B, Hakes T, et al. Evidence supporting the superiority of intraperitoneal cisplatin compared to intraperitoneal carboplatin for salvage therapy of small-volume residual ovarian cancer. Gynecol Oncol 1993; 50(1): 100–4
Fujiwara K, Sakuragi N, Suzuki S, et al. First-line intraperitoneal carboplatin-based chemotherapy for 165 patients with epithelial ovarian carcinoma: results of long-term follow-up. Gynecol Oncol 2003; 90: 637–43
Zylberberg B, Ravina JH, Salat-Baroux J, et al. Polychemotherapy of ovarian cancer via combined intravenous and intraperitoneal routes: technic and preliminary results. J Gynecol Obstet Biol Reprod (Paris) 1986; 15(5): 671–6
Kirmani S, Braly PS, McClay EF, et al. A comparison of intravenous versus intraperitoneal chemotherapy for the initial treatment of ovarian cancer. Gynecol Oncol 1994; 54: 338–44
Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 1996; 335: 1950–5
Polyzos A, Tsavaris N, Kosmas C, et al. A comparative study of intraperitoneal carboplatin versus intravenous carboplatin with intravenous cyclophosphamide in both arms as initial chemotherapy for stage III ovarian cancer. Oncology 1999; 56: 291–6
Gadducci A, Carnino F, Chiara S, et al. Intraperitoneal versus intravenous cisplatin in combination with intravenous cyclophosphamide and epidoxorubicin in optimally cytoreduced advanced epithelial ovarian cancer: a randomized trial of the Gruppo Oncologico Nord-Ovest. Gynecol Oncol 2000; 76: 157–62
Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 2001; 19: 1001–7
Yen MS, Juang CM, Lai CR, et al. Intraperitoneal cisplatin-based chemotherapy vs. intravenous cisplatin-based chemotherapy for stage III optimally cytoreduced epithelial ovarian cancer. Int J Gynaecol Obstet 2001; 72: 55–60
Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006; 354: 34–43
Elit L, Oliver TK, Covens A, et al. Intraperitoneal chemotherapy in the first-line treatment of women with stage III epithelial ovarian cancer: a systemic review with metaanalyses. Cancer 2007; 109: 692–702
Jaaback K, Johnson N. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev 2006; (1): CD005340
National Cancer Institute. NCI clinical announcement: intraperitoneal chemotherapy for ovarian cancer. Bethesda (MD): National Cancer Institute, 2006 Jan 5 [online]. Available from URL: http://ctep.cancer.gov/highlights/docs/clin_annc_010506.pdf [Accessed 2012 Feb 15]
Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 2003; 21: 3194–200
Piccart MJ, Floquet A, Scarfone G, et al. Intraperitoneal cisplatin versus no further treatment: 8-year results of EORTC 55875, a randomized phase III study in ovarian cancer patients with a pathologically complete remission after platinum-based intravenous chemotherapy. Int J Gynecol Cancer 2003; 13 Suppl. 2: 196–203
Marth C, Walker JL, Barakat RR, et al. Results of the 2006 Innsbruck International Consensus Conference on intraperitoneal chemotherapy in patients with ovarian cancer. Cancer 2007; 109(4): 645–9
NCIC Clinical Trials Group. A phase II/III study of intraperitoneal (IP) plus intravenous (IV) chemotherapy versus IV carboplatin plus paclitaxel in patients with epithelial ovarian cancer optimally debulked at surgery following neoadjuvant intravenous chemotherapy [Clinicaltrials.gov identifier NCT00993655]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov/show/NCT00993655 [Accessed 2011 Aug 26]
Gynecologic Oncology Group. A phase III clinical trial of bevacizumab with IV versus IP chemotherapy in ovarian, fallopian tube, and primary peritoneal carcinoma [Clinicaltrials.gov identifier NCT00951496]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov/show/NCT00951496 [Accessed 2011 Aug 26]
Gaillard S, Armstrong D. Intraperitoneal chemotherapy for advanced epithelial ovarian cancer: many questions, much promise... Oncology 2011; 25: 176, 178, 180
de Gramont A, Demuynck B, Louvet C, et al. Survival with intraperitoneal cisplatin in advanced ovarian cancer after second-look laparotomy. Am J Clin Oncol 1992; 15:7–11
Dufour P, Bergerat JP, Barats JC, et al. Intraperitoneal mitoxantrone as consolidation treatment for patients with ovarian carcinoma in pathologic complete remission. Cancer 1994; 73: 1865–9
Menczer J, Ben-Baruch G, Rizel S, et al. Intraperitoneal cisplatin chemotherapy in ovarian carcinoma patients who are clinically in complete remission. Gynecol Oncol 1992; 46: 222–5
Tarraza Jr HM, Boyce CR, Smith WG, et al. Consolidation intraperitoneal chemotherapy in epithelial ovarian cancer patients following negative second-look laparotomy. Gynecol Oncol 1993; 50: 287–90
Topuz E, Eralp Y, Saglam S, et al. Efficacy of intraperitoneal cisplatin as consolidation therapy in patients with pathologic complete remission following front-line therapy for epithelial ovarian cancer: consolidative intraperitoneal cisplatin in ovarian cancer. Gynecol Oncol 2004; 92: 147–51
Tournigand C, Louvet C, Molitor JL, et al. Long-term survival with consolidation intraperitoneal chemotherapy for patients with advanced ovarian cancer with pathological complete remission. Gynecol Oncol 2003; 91: 341–5
Barakat RR, Sabbatini P, Bhaskaran D, et al. Intraperitoneal chemotherapy for ovarian carcinoma: results of long-term follow-up. J Clin Oncol 2002; 20: 694–8
Howell SB, Zimm S, Markman M, et al. Long-term survival of advanced refractory ovarian carcinoma patients with small-volume disease treated with intraperitoneal chemotherapy. J Clin Oncol 1987; 5: 1607–12
Markman M, Berek JS, Blessing JA, et al. Characteristics of patients with small-volume residual ovarian cancer unresponsive to cisplatin-based IP chemotherapy: lessons learned from a Gynecologic Oncology Group phase II trial of IP cisplatin and recombinant alpha-interferon. Gynecol Oncol 1992; 45: 3–8
Markman M, Reichman B, Hakes T, et al. Responses to second-line cisplatin based intraperitoneal therapy in ovarian cancer: influence of a prior response to intravenous cisplatin. J Clin Oncol 1991; 9: 1801–5
Markman M, Blessing JA, Major F, et al. Salvage intraperitoneal therapy of ovarian cancer employing cisplatin and etoposide: a Gynecologic Oncology Group study. Gynecol Oncol 1993; 50: 191–5
Berek JS, Markman M, Blessing JA, et al. Intraperitoneal α-interferon alternating with cisplatin in residual ovarian carcinoma: a phase II Gynecologic Oncology Group study. Gynecol Oncol 1999; 74: 48–52
Berek JS, Markman M, Stonebraker B, et al. Intraperitoneal interferon-α in residual ovarian carcinoma: a phase II Gynecologic Oncology Group study. Gynecol Oncol 1999; 75: 10–4
Muggia FM, Groshen S, Russell C, et al. Intraperitoneal carboplatin and etoposide for persistent epithelial ovarian cancer: analysis of results by prior sensitivity to platinum-based regimens. Gynecol Oncol 1993; 50: 232–8
Bruzzone M, Rubagotti A, Gadducci A, et al. Intraperitoneal carboplatin with or without interferon-alpha in advanced ovarian cancer patients with minimal residual disease at second look: a prospective randomized trial of 111 patients. Gynecol Oncol 1997; 65: 499–505
Minsky BD, Mies C, Rich TA, et al. Potential curative surgery of colon cancer: patterns of failure and survival. J Clin Oncol 1988; 6: 106–18
Brodsky JT, Cohen AM. Peritoneal seeding following potentially curative resection of colonic carcinoma: implications for adjuvant therapy. Dis Colon Rectum 1991; 34: 723–7
Dawson LE, Russell AH, Tong D, et al. Adenocarcinoma of the sigmoid colon: sites of initial dissemination and clinical patterns of recurrence following surgery alone. J Surg Oncol 1983; 22: 95–9
Tong D, Russell AH, Dawson LE, et al. Adenocarcinoma of the cecum: natural history and clinical patterns of recurrence following radical surgery. Int J Radiat Oncol Biol Phys 1983; 9: 357–60
Russell AH, Tong D, Dawson LE, et al. Adenocarcinoma of the retro-peritoneal ascending and descending colon: sites of initial dissemination and clinical patterns of recurrence following surgery alone. Int J Radiat Oncol Biol Phys 1983; 9: 361–5
Sugarbaker PH. Intraperitoneal chemotherapy for treatment and prevention of peritoneal carcinomatosis and sarcomatosis. Dis Colon Rectum 1994; 37 (Suppl.): S115–22
Sugarbaker PH, Gianola FJ, Spreyer JL, et al. Prospective randomized trial of intravenous versus intraperitoneal 5-fluorouracil in patients with advanced primary colon or rectal cancer. Surgery 1985; 98: 414–21
Scheithauer W, Kornek GV, Marczell A, et al. Combined intravenous and intraperitoneal chemotherapy with fluorouracil + leucovorin vs fluorouracil + levamisole for adjuvant therapy of resected colon carcinoma. Br J Cancer 1998; 77: 1349–54
Vaillant J-C, Nordlinger B, Deuffic S, et al. Adjuvant intraperitoneal 5-fluorouracil in high-risk colon cancer: a multicenter phase III trial. Ann Surg 2000; 231: 449–56
Nordlinger B, Rougier P, Arnaud JP, et al. Adjuvant regional chemotherapy and systemic chemotherapy versus systemic chemotherapy alone in patients with stage II–III colorectal cancer: a multicentre randomised controlled phase III trial. Lancet Oncol 2005; 6: 459–68
De Bree E, Witkamp AJ, Zoetmulder AN. Intraperitoneal chemotherapy for colorectal cancer. J Surg Oncol 2002; 79: 46–61
Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg 1995; 221: 124–32
Sugarbaker PH, Schellinx MET, Chang D, et al. Peritoneal carcinomatosis from adenocarcinoma of the colon. World J Surg 1996; 20: 585–92
Sugarbaker PH. Treatment of peritoneal carcinomatosis from colon or appendiceal cancer with induction intraperitoneal chemotherapy. Cancer Treat Res 1996; 82: 317–25
Yano M, Yasuda T, Fujiwara Y, et al. Preoperative intraperitoneal chemotherapy for patients with serosa-infiltrating gastric cancer. J Surg Oncol 2004; 88: 39–43
Yonemura Y, Bandou E, Sawa T, et al. Neoadjuvant treatment of gastric cancer with peritoneal dissemination. Eur J Surg Oncol 2006; 32: 661–5
Yonemura Y, Endou Y, Shinbo M, et al. Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: selection for cytoreductive surgery. J Surg Oncol 2009; 100: 311–6
Matharu G, Tucker O, Alderson D. Systematic review of intraperitoneal chemotherapy for gastric cancer. Br J Surg 2001; 98: 1225–35
Cheong JH, Shen JY, Song CS, et al. Early postoperative intraperitoneal chemotherapy following cytoreductive surgery in patients with very advanced gastric cancer. Ann Surg Oncol 2006; 14: 61–8
Brenner B, Shah MA, Karpeh MS, et al. A phase II trial of neoadjuvant cisplatin-fluorouracil followed by postoperative intraperitoneal floxuridine-leucovorin in patients with locally advanced gastric cancer. Ann Oncol 2006; 17: 1404–11
Newman E, Potmesil M, Ryan T, et al. Neoadjuvant chemotherapy, surgery, and adjuvant intraperitoneal chemotherapy in patients with locally advanced gastric or gastroesophageal junction carcinoma: a phase II study. Semin Oncol 2005; 32: S97–100
Ishigami H, Kitayama J, Kaisaki S, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol 2010; 21: 67–70
Walker JL, Armstrong DK, Huang HQ, et al. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: a Gynecologic Oncology Group study. Gynecol Oncol 2006; 100: 27–32
Piccart MJ, Speyer JL, Markman M, et al. Intraperitoneal chemotherapy: technical experience at five institutions. Semin Oncol 1985; 12: 90–6
Spratt JS, Adcock RA, Muskovin M, et al. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res 1980; 40: 256–60
Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer 2008; 44:2546–54
Los G, Sminia P, Wondergem J, et al. Optimization of intraperitoneal cisplatin therapy with regional hyperthermia in rats. Eur J Cancer 1991; 27: 472–7
Los G, Smals OA, van Vugt MJ, et al. A rationale for carboplatin treatment and abdominal hyperthermia in cancers restricted to the peritoneal cavity. Cancer Res 1992; 52: 1252–8
Jacquet P, Averbach A, Stuart OA, et al. Hyperthermic intraperitoneal doxorubicin: pharmacokinetics, metabolism, and tissue distribution in a rat model. Cancer Chemother Pharmacol 1998; 41: 147–54
Jacquet P, Averbach A, Stephens AD, et al. Heated intraoperative intraperitoneal mitomycin C and early postoperative intraperitoneal 5-fluorouracil: pharmacokinetic studies. Oncology 1998; 55(2): 130–8
The Netherlands Cancer Institute. Phase III randomised clinical trial for stage III ovarian carcinoma randomising between secondary debulking surgery with or without hyperthermic intraperitoneal chemotherapy [Clinical-Trials.gov identifier NCT00426257]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov/show/NCT00426257 [Accessed 2011 Aug 26]
Franko J, Ibrahim Z, Gusani N, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer 2010; 116: 2756–62
Zhu ZG, Tang R, Yan M, et al. Efficacy and safety of intraoperative peritoneal hyperthermic chemotherapy for advanced gastric cancer patients with serosal invasion: a long-term follow-up study. Dig Surg 2006; 23: 93–102
Hall JJ, Loggie BW, Shen P, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg 2004; 8: 454–63
Glehen O, Schreiber V, Cotte E, et al. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg 2004; 139: 20–6
Yonemura Y, Kawamura T, Bandou E, et al. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 2005; 92: 370–5
Scaringi S, Kianmanesh R, Sabate JM, et al. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: a single western center experience. Eur J Surg Oncol 2008; 34: 1246–52
Yan TD, Black D, Sugarbaker PH, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol 2007; 14(10): 2702–13
Yan TD, Welch L, Black D, et al. A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignancy peritoneal mesothelioma. Ann Oncol 2007; 18: 827–34
Yan TD, Links M, Xu ZY, et al. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei from appendiceal mucinous neoplasms. Br J Surg 2006; 93: 1270–6
Elias D, Honore C, Ciuchendea R, et al. Peritoneal pseudomyxoma: results of a systematic policy of complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Br J Surg 2008; 95: 1164–71
Mohamed F, Moran BJ. Morbidity and mortality with cytoreductive surgery and intraperitoneal chemotherapy. Cancer J 2009; 15: 196–9
Chua TC, Yan TD, Saxena A, et al. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure. Ann Surg 2009; 249: 900–7
Loungnarath R, Causeret S, Bossard N, et al. Cytoreductive surgery with intraperitoneal chemohyperthermia for the treatment of pseudomyxoma peritonei: a prospective study. Dis Colon Rectum 2005; 48: 1372–9
Witkamp AJ, de Bree E, Kaag MM, et al. Extensive surgical cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in patients with pseudomyxoma peritonei. Br J Surg 2001; 88: 458–63
Moran BJ, Mukherjee A, Sexton R. Operability and early outcome in 100 consecutive laparotomies for peritoneal malignancy. Br J Surg 2006; 93: 100–4
Murphy EM, Sexton R, Moran BJ. Early results of surgery in 123 patients with pseudomyxoma peritonei from a perforated appendiceal neoplasm. Dis Colon Rectum 2007; 50: 37–42
Butterworth SA, Panton ON, Klaassen DJ, et al. Morbidity and mortality associated with intraperitoneal chemotherapy for Pseudomyxoma peritonei. Am J Surg 2002; 183: 529–32
Smeenk RM, Verwaal VJ, Zoetmulder FA. Toxicity and mortality of cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei - a report of 103 procedures. Eur J Surg Oncol 2006; 32: 186–90
Glehen O, Osinsky D, Cotte E, et al. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol 2003; 10: 863–9
Jacquet P, Stephens AD, Averbach AM, et al. Analysis of morbidity and mortality in 60 patients with peritoneal carcinomatosis treated by cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy. Cancer 1996; 77: 2622–9
Stephens AD, Alderman R, Chang D, et al. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol 1999; 6: 790–6
Madhusudan S, Tamir A, Bates N, et al. A multicenter phase I gene therapy clinical trial involving intraperitoneal administration of E1A-lipid complex in patients with recurrent epithelial ovarian cancer overexpressing HER-2/neu oncogene. Clin Cancer Res 2004; 10: 2986–96
Berek JS, Hacker NF, Lichtenstein A, et al. Intraperitoneal recombinant alpha-interferon for “salvage” immunotherapy in stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Cancer Res 1985; 45:4447–53
Nicoletto MO, Fiorentino MV, Vinante O, et al. Experience with intraperitoneal alpha-2a interferon. Oncology 1992; 49: 467–73
Aarts F, Bleichrodt RP, Oyen WJ, et al. Intracavitary radio immunotherapy to treat solid tumors. Cancer Biother Radiopharm 2008; 23: 92–107
Koppe MJ, Hendriks T, Boerman OC, et al. Radioimmunotherapy is an effective adjuvant treatment after cytoreductive surgery of experimental colonic peritoneal carcinomatosis. J Nucl Med 2006; 47: 1867–74
Meredith RF, Buchsbaum DJ, Alvarez RD, et al. Brief overview of preclinical and clinical studies in the development of intraperitoneal radioimmunotherapy for ovarian cancer. Clin Cancer Res 2007; 13: 5643s–5s
Sebastian M, Kuemmel A, Schmidt M, et al. Catumaxomab: a biphasic trifunctional antibody. Drugs Today 2001; 45: 589–97
Linke R, Klein A, Seimetz D. Catumaxomab: clinical development and future directions. MAbs 2010; 2: 129–36
Acknowledgements
The authors have no conflicts of interest or funding sources to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hasovits, C., Clarke, S. Pharmacokinetics and Pharmacodynamics of Intraperitoneal Cancer Chemotherapeutics. Clin Pharmacokinet 51, 203–224 (2012). https://doi.org/10.2165/11598890-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11598890-000000000-00000