Abstract
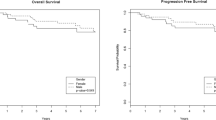
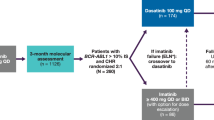
Dasatinib is an oral dual tyrosine kinase inhibitor active against ABL1 and SRC family kinases. The US FDA approved it for the treatment of chronic myeloid leukemia (CML) patients in chronic, accelerated, or blastic phase with resistance or intolerance to imatinib therapy. Dasatinib is also indicated for the treatment of adults with Philadelphia chromosome-positive acute lymphoblastic leukemia who have become resistant to or intolerant of other treatments. The agent is now also approved for newly diagnosed chronic phase (CP) patients. This article reviews the pharmacokinetic and pharmacodynamic properties of dasatinib as well as clinical data limited to CP-CML patients. Four-year follow-up of a phase III dose-optimization trial confirmed that better progression-free survival (66%) and overall survival (82%) were obtained with a dose of 100 mg once daily (od) than with the standard 70 mg twice daily dosing (65% and 75%, respectively). The 100 mg od dosing schedule was also associated with the highest benefit-risk ratio for CP patients with resistant, intolerant, or suboptimal response. Recent results of a phase III trial in newly diagnosed patients demonstrated that dasatinib 100 mg od has superiority in terms of confirmed cytogenetic and molecular responses, with faster responses and high activity in high Sokal risk patients compared with standard-dose imatinib.




Similar content being viewed by others
References
Goldman JM, Melo JV. Chronic myeloid leukemia: advances in biology and new approaches to treatment. N Engl J Med 2003; 349: 1451–64
Hantschel O, Superti-Furga G. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol Cell Biol 2004; 5: 33–44
Hehlmann R, Hochhaus A, Baccarani M. European LeukemiaNet: chronic myeloid leukaemia. Lancet 2007; 370: 342–50
Faderl S, Talpaz M, Estrov Z, et al. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med 1999; 131: 207–19
Deininger MW, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow-up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib [abstract]. Blood 2009; 114: 1126
Hughes TP, Hochhaus A, Branford S, et al. Reduction of BCR-ABL transcript levels at 6, 12, and 18 months (mo) correlates with long-term outcomes on imatinib (IM) at 72 mo: an analysis from the International Randomized Study of Interferon versus STI571 (IRIS) in Patients (pts) with Chronic Phase Chronic Myeloid Leukemia (CML-CP) [abstract]. Blood 2008; 112: 1109
Apperley JF. Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol 2007; 8: 1018–29
Milojkovic D, Apperley JF. Mechanisms of resistance to imatinib and second-generation tyrosine inhibitors in chronic myeloid leukaemia. Clin Cancer Res 2009; 15: 7519–27
Breccia M, Alimena G. Resistance to imatinib in chronic myeloid leukemia and therapeutic approaches to circumvent the problem. Cardiovasc Hematol Disord Drug Targets 2009; 9: 21–8
Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 2006; 108: 1809–20
Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol 2009; 27: 6041–51
Branford S, Rudzki Z, Walsh S, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance and mutations in the ATP phosphate-binding loop (p-loop) are associated with a poor prognosis. Blood 2003; 102: 276–83
Jabbour E, Kantarjian H, Jones D, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukaemia treated with imatinib mesylate. Leukemia 2006; 20: 1767–73
Soverini S, Colarossi S, Gnani A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res 2006; 12: 7374–9
Nicolini FE, Corm S, Le QH, et al. Mutation status and clinical outcome of 89 imatinib mesylate-resistant chronic myelogenous leukaemia patients: a retrospective analysis from the French intergroup of CML. Leukemia 2006; 20: 1061–6
Sherbenou DW, Wong MJ, Humayun A, et al. Mutations of the BCR-ABL kinase domain occur in a minority of patients with stable complete cytogenetic response to imatinib. Leukemia 2007; 21: 489–93
Khorashad JS, deLavallade H, Apperley JF, et al. Finding of kinase domain mutations in patients with chronic phase chronic myeloid leukaemia responding to imatinib may identify those at high risk of disease progression. J Clin Oncol 2008; 26: 4806–13
Keam SJ. Dasatinib: in chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. BioDrugs 2008; 22: 59–69
Jabbour E, Cortes J, Kantarjian H. Dasatinib for the treatment of Philadelphia chromosome-positive leukemias. Expert Opin Investig Drugs 2007; 16: 679–87
Steinberg M. Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukaemia and Philadelphia chromosome positive acute lymphoblastic leukaemia. Clin Ther 2007; 29: 2289–308
Shah NP. Dasatinib. Drugs Today (Barc) 2007; 43: 5–12
Nam S, Williams A, Vultur A, et al. Dasatinib (BMS-354825) inhibits Stat5 signaling associated with apoptosis in chronic myelogenous leukemia cells. Mol Cancer Ther 2007; 6: 1400–5
La Rosee P, Hartel N, Klag T, et al. MAPK-activation in response to BCR-ABL inhibition is cytokine-dependent and can be overcome by dasatinib [abstract]. Haematologica 2007; 92 Suppl. 1: 199
Lombardo LJ, Lee FY, Chen P, et al. Discovery of N- (2-chloro-6-methylphenyl)-2- (6 (4- (2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS 354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem 2004; 47: 6658–61
Donato NJ, Wu J, Kong LY, et al. The SRC/ABL inhibitor BMS-354825 overcomes resistance to imatinib mesylate in chronic myelogenous leukemia cells through multiple mechanisms [abstract]. Blood 2004; 104: 549
Dasatinib: summary of product characteristics. Uxbridge: Bristol-Myers Squibb Pharma EEIG, 2007
Kaul S, Wu C, Mayfield S, et al. Dasatinib can be administered orally with or without meal [abstract]. Blood 2007; 110: 4569
Wu CY, Callegari F, McBann B, et al. Mass balance, pharmacokinetics and metabolism of [14C] BMS-354825 in healthy male subjects [abstract]. EJC 2006; 4: 50
Wu CY, Callegari F, Williams K, et al. Effects of dasatinib on the pharmacokinetics of sinvastatin, a cytochrome P450 3A4 substrate, in healthy subjects. EJC 2006; 4: 48–9
Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med 2006; 354: 2531–41
Cortes J, Sawyers CL, Kantarjian H, et al. Long-term efficacy of dasatinib in chronic phase CML: results from the phase I trial (CA180002) [abstract]. Blood 2007; 110: 1026
Hochhaus A, Kantarjian H, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic phase chronic myeloid leukemia after failure of imatinib therapy. Blood 2007; 109: 2303–9
Hochhaus A, Baccarani M, Deiniger M, et al. Dasatinib induces durable cytogentic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia 2008; 22: 1200–6
Kantarjian H, Pasquini R, Levy V, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia resistant to imatinib at a dose of 400 to 600 milligrams daily: two-year follow-up of a randomized phase 2 study (START-R). Cancer 2009; 115: 3935–43
Shah NP, Cortes J, Schiffer C, et al. Four-year follow-up of patients with chronic-phase chronic myeloid leukemia (CP-CML) receiving dasatinib 100 mg once daily [abstract]. J Clin Oncol 2010; 28: 6512
Pavlù J, Marin D. Dasatinib and chronic myeloid leukemia: two-year follow up in eight clinical trials. Clin Lymphoma Myeloma 2009; 9: 417–24
Branford S, Hochhaus A, Mueller M, et al. Analysis of molecular data and the emergence of mutations for chronic-phase chronic myelogenous leukemia (CML-CP) patients treated with dasatinib after imatinib failure [abstract]. Blood 2009; 114: 3282
Saglio G, Cortes JE, Schiffer CA, et al. Types of resistance to imatinib and other potential predictors of response to second-line dasatinib therapy [abstract]. J Clin Oncol 2010; 28: 6569
Roy A, Shah NP, le Coutre P, et al. Relationship between dasatinib exposure and long-term clinical outcomes in chronic phase chronic myeloid leukemia (CML-CP) patients [abstract]. J Clin Oncol 2010; 28: 6518
Muller MC, Cortes JE, Kim DW, et al. Dasatinib treatment of chronic phase chronic myeloid leukemia: analysis of responses according to pre-existing BCR-ABL mutations. Blood 2009; 114: 4944–53
Quintas-Cardama A, Kantarjian H, O’Brien S, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol 2007; 25: 3908–14
de Lavallade H, Punnialingam S, Milojkovic D, et al. Pleural effusions in patients with chronic myeloid leukemia treated with dasatinib may have an immune-mediated pathogenesis. Br J Haematol 2008; 141: 745–7
Breccia M, Alimena G. Pleural/pericardic effusions during dasatinib treatment: incidence, management and risk factors associated to their development. Expert Opin Drug Saf 2010; 9: 713–21
Porkka K, Khoury HJ, Paquette RL, et al. Dasatinib 100 mg once daily minimizes the occurrence of pleural effusion in patients with chronic myeloid leukemia in chronic phase and efficacy is unaffected in patients who develop pleural effusion. Cancer 2010; 116: 377–86
Pinilla-Ibarz J, Cortes J, Mauro MJ. Intolerance to tyrosine kinase inhibitors in chronic myeloid leukemia. Cancer 2010; 117: 688–97
Mustjoki S, Ekblom M, Arstila TP, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia 2009; 23: 1398–405
Mustjoki S, Kreutzman A, Dix C, et al. Large granular lymphocyte (LGL) expansions comprising oligiclonal T cell or NK cell populations in dasatinib treated patients are associated with HLA-A 0201, CMV reactivation and enhanced anti-leukemic control [abstract]. Blood 2009; 114: 1123
Hiwase DK, Saunders V, Hewett D, et al. Dasatinib cellular uptake and efflux in chronic myeloid leukemia cells: therapeutic implications. Clin Cancer Res 2008; 14: 3881–8
Lagas JS, vanWaterschoot RA, van Tilburg VA, et al. Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clin Cancer Res 2009; 15: 2344–51
Porkka K, Koskenvesa P, Lundan T, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood 2008; 112: 1005–12
Fei F, Yu Y, Schmitt A, et al. Dasatinib inhibits the proliferation and function of CD4+CD25+ regulatory T cells. Br J Haematol 2009; 144: 195–205
Breccia M, Palandri F, Iori AP, et al. Second-generation tyrosine kinase inhibitors before allogeneic stem cell transplantation in patients with chronic myeloid leukemia resistant to imatinib. Leuk Res 2010; 34: 143–7
Klyuchnikov E, Kroger N, Brummendorf TH, et al. Current status and perspectives of tyrosine kinase inhibitor treatment in the post-transplant period in patients with chronic myelogenous leukemia (CML). Biol Blood Marrow Transplant 2010; 16: 301–10
Steegman JL, Michallet M, Morra E, et al. A European observational study of dasatinib in the management of imatinib-resistant and intolerant patients with chronic myeloid leukemia: FORTE study (CA180-211) [abstract]. Haematologica 2010; 95: 810
Cortes J, Jones D, O’Brien S, et al. Results of dasatinib therapy in patients with early chronic phase chronic myeloid leukemia. J Clin Oncol 2010; 28: 398–404
Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010; 362: 2260–70
Radich JP, Kopecky KJ, Kamel-Reid S, et al. A randomized phase II trial of dasatinib 100 mg vs imatinib 400 mg in newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): the S0325 Intergroup trial [abstract]. Blood 2010; 116: 6
Rousselot P, Bouchet S, Etienne G, et al. Early cytogenetic and molecular responses and pharmacokinetic of dasatinib as first line therapy in newly diagnosed chronic phase CML patients: first analysis of the OPTIM dasatinib trial [abstract]. Haematologica 2010; 95: 1139
Acknowledgments
No sources of funding were used to assist in the preparation of this manuscript. M. Breccia wrote the paper and reviewed the literature and G. Alimena approved the final version of the paper. G. Alimena and M. Breccia have received consultancies and honoraria from Novartis Pharma and Bristol-Myers Squibb. We thank Elisabetta Verrillo of inScience Communications, a Wolters Kluwer business, who provided assistance with the submission of this manuscript. This assistance was funded by Bristol-Myers Squibb S.r.l.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Breccia, M., Alimena, G. Activity and Safety of Dasatinib as Second-Line Treatment or in Newly Diagnosed Chronic Phase Chronic Myeloid Leukemia Patients. BioDrugs 25, 147–157 (2011). https://doi.org/10.2165/11591840-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11591840-000000000-00000