Abstract
Interferon-β-1b has been used as a disease-modifying therapy in multiple sclerosis (MS) for many years. Although its mechanism of action in MS has not been fully elucidated, it appears to involve immunomodulatory effects mediated by interactions with specific receptors.
Large, randomized, multicentre, clinical trials of 2–3.5 years’ duration have demonstrated the efficacy of interferon-β-1b 250 μg subcutaneously every other day in patients with a first clinical event suggestive of MS (i.e. those with clinically isolated syndrome [CIS]) and in those with relapsing-remitting MS (RRMS). In terms of its efficacy on primary (or co-primary) endpoints, interferon-β-1b significantly reduced the risk of developing clinically definite MS compared with placebo in patients with CIS in the BENEFIT study. In patients with RRMS, interferon-β-1b was associated with a significantly lower annualized relapse rate and a significantly higher proportion of relapse-free patients compared with placebo in a registration trial conducted by the Interferon-β MS Study Group. The INCOMIN trial in patients with RRMS showed a significant advantage of interferon-β-1b over intramuscular interferon-β-1a in terms of the percentage of relapse- and progression-free patients and the proportion of patients without new MRI-documented lesions. Other active-comparator trials in RRMS used a variety of primary (or co-primary) endpoints and showed no significant differences between interferon-β-1b and either subcutaneous glatiramer acetate (BECOME and BEYOND trials) or subcutaneous interferon-β-1a (Danish MS Group trial) for these outcomes.
In patients with secondary progressive MS (SPMS), the European Study Group showed that interferon-β-1b significantly increased the time to confirmed disease progression compared with placebo, although there was no significant between-group difference for this primary endpoint in a similar trial conducted by the North American Study Group. The studies allowed inclusion of patients with superimposed relapse, and both trials showed a significant reduction in annualized relapse rate with interferon-β-1b.
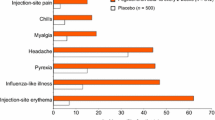
The most frequently reported adverse events with interferon-β-1b are flu-like symptoms and injection-site reactions, which can usually be managed. The incidence of these adverse events generally declines markedly after the first year of treatment. Lymphopenia is the most frequently reported laboratory abnormality and occurs in the majority of patients. Depression, suicidal ideation and injectionsite necrosis were the most serious adverse events reported with interferon-β-1b in clinical trials. Long-term safety data over a 16-year follow-up period showed no unexpected adverse events among patients treated with interferon-β-1b.
Thus, interferon-β-1b is a well established, first-line, disease-modifying therapy that has demonstrated efficacy in newly emerging MS, RRMS and SPMS with superimposed relapse in well designed clinical trials, and has a generally manageable tolerability profile, with no unexpected adverse events after many years of follow-up.






Similar content being viewed by others
References
World Health Organization, Multiple Sclerosis International Federation. Atlas: multiple sclerosis resources in the world 2008 [online]. Available from URL: http://www.msif.org/en/about_msif/what_we_do/atlas_of_ms/ [Accessed 2010 Jul 30]
National Multiple Sclerosis Society. Who gets MS? [online]. Available from URL: http://www.nationalmssociety.org/about-multiple-slcerosis/what-we-know-about-ms/who-getsms/index.aspx [Accessed 2010 Jul 30]
Weiner HL. The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease? Ann Neurol 2009; 65: 239–48
Pugliatti M, Rosati G, Carton H, et al. The epidemiology of multiple sclerosis in Europe. Eur J Neurol 2006; 13: 700–22
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996 Apr; 46(4): 907–11
Confavreux C, Vukusic S, Moreau T, et al. Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000 Nov 16; 343(20): 1430–8
Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 1989 Feb; 112 Pt 1: 133–46
Leary SM, Thompson AJ. Primary progressive multiple sclerosis: current and future treatment options. CNS Drugs 2005; 19(5): 369–76
Thompson AJ, Montalban X, Barkhof F, et al. Diagnostic criteria for primary progressive multiple sclerosis: a position paper. Ann Neurol 2000 Jun; 47(6): 831–5
Comi G. Shifting the paradigm toward earlier treatment of multiple sclerosis with interferon beta. Clin Ther 2009 Jun; 31(6): 1142–57
Stuve O, Bennett JL, Hemmer B, et al. Pharmacological treatment of early multiple sclerosis. Drugs 2008; 68(1): 73–83
Freedman MS, Hughes B, Mikol DD, et al. Efficacy of disease-modifying therapies in relapsing remitting multiple sclerosis: a systematic comparison. Eur Neurol 2008; 60(1): 1–11
Goodin DS. Disease-modifying therapy in multiple sclerosis: update and clinical implications. Neurology 2008 Dec 9; 71(24 Suppl. 3): S8–13
Goodin DS, Frohman EM, Garmany Jr GP, et al. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology 2002 Jan 22; 58(2): 169–78
Giovannoni G. Management of secondary-progressive multiple sclerosis. CNS Drugs 2004; 18(10): 653–69
McKeage K. Interferon-beta-1b: in newly emerging multiple sclerosis. CNS Drugs 2008; 22(9): 787–92
McCormack PL, Scott LJ. Interferon-beta-1b: a review of its use in relapsing-remitting and secondary progressive multiple sclerosis. CNS Drugs 2004; 18(8): 521–46
Dhib-Jalbut S. Mechanisms of action of interferons and glatiramer acetate in multiple sclerosis. Neurology 2002 Apr 23; 58(8 Suppl. 4): S3–9
Bayer HealthCare — Bayer Schering Pharma. Betaferon® (recombinant interferon beta-1b powder and solvent for solution for injection): summary of product characteristics [online]. Available from URL: http://www.medicines.org.uk/EMC/medicine/1809/SPC/Betaferon [Accessed 2010 Jul 20]
Bayer HealthCare Pharmaceuticals. Betaseron® (interferon beta-1b for subcutaneous injection): US prescribing information [online]. Available from URL: http://berlex.bayerhealthcare.com/html/products/pi/Betaseron_PI.pdf [Accessed 2010 Jul 29]
Williams GJ, Witt PL. Comparative study of the pharmacodynamic and pharmacologic effects of Betaseron and Avonex. J Interferon Cytokine Res 1998 Nov; 18(11): 967–75
Bertolotto A, Gilli F, Sala A, et al. Evaluation of bioavailability of three types of IFN-beta in multiple sclerosis patients by a new quantitative-competitive-PCR method for MxA quantification. J Immunol Methods 2001; 256(1–2): 141–52
Sturzebecher S, Maibauer R, Heuner A, et al. Pharmacodynamic comparison of single doses of IFN-beta1a and IFN-beta1b in healthy volunteers. J Interferon Cytokine Res 1999; 19(11): 1257–2126
Malucchi S, Gilli F, Caldano M, et al. Predictive markers for response to interferon therapy in patients with multiple sclerosis. Neurology 2008 Mar 2; 70(13): 1119–27
Deisenhammer F. Neutralizing antibodies to interferon-beta and other immunological treatments for multiple sclerosis: prevalence and impact on outcomes. CNS Drugs 2009; 23(5): 379–96
Goodin DS, Frohman EM, Hurwitz B, et al. Neutralizing antibodies to interferon beta: assessment of their clinical and radiographic impact: an evidence report. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2007 Mar 27; 68(13): 977–84
European Study Group on Interferon beta-1b in Secondary Progressive MS. Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. Lancet 1998 Nov 7; 352: 1491–7
Durelli L, Verdun E, Barbero P, et al. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN). Lancet 2002 Apr 27; 359(9316): 1453–60
Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 2006 Oct 10; 67(7): 1242–9
North American Study Group on Interferon beta-1b in Secondary Progressive MS. Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology 2004 Nov 23; 63(10): 1788–95
The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology 1993 Apr; 43(4): 655–61
Sorensen PS, Ross C, Clemmesen KM, et al. Clinical importance of neutralising antibodies against interferon beta in patients with relapsing-remitting multiple sclerosis. Lancet 2003 Oct 11; 362(9391): 1184–91
Chiang J, Gloff CA, Yoshizawa CN, et al. Pharmacokinetics of recombinant human interferon-beta ser in healthy volunteers and its effect on serum neopterin. Pharm Res 1993 Apr; 10(4): 567–72
Kurtzke JF. Rating neurological impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 1983 Nov; 33(11): 1444–52
Kappos L, Freedman MS, Polman CH, et al. Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet 2007 Aug 4; 370(9585): 389–97
Kappos L, Freedman MS, Polman CH, et al. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol 2009 Nov; 8(11): 987–97
Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983; 13: 227–31
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50: 121–7
Barkhof F, Polman CH, Radue EW, et al. Magnetic resonance imaging effects of interferon beta-1b in the BENEFIT study: integrated 2-year results. Arch Neurol 2007 Sep; 64(9): 1292–8
Sahraian MA, Radue EW, Haller S, et al. Black holes in multiple sclerosis: definition, evolution, and clinical correlations. Acta Neurol Scand 2010 Jul; 122(1): 1–8
Foley F, Benedict R, Penner I-K, et al. Treatment effects of IFNB-1b on paced auditory serial addition test performance in patients with a first event suggestive of MS [abstract no. P-807 plus poster]. 26th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); 2010 Oct 13–16; Gothenburg
Koch-Henriksen N, Sorensen PS, Christensen T, et al. A randomized study of two interferon-beta treatments in relapsing-remitting multiple sclerosis. Neurology 2006 Apr 11; 66(7): 1056–60
O’Connor P, Filippi M, Arnason B, et al. 250 ug or 500 ug interferon beta-1b versus 20mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol 2009 Oct; 8(10): 889–97
Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology 2009 Jun 9; 72(23): 1976–83
Paty DW, Li DK. Interferon beta-1b is effective in relapsingremitting multiple sclerosis: II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology 1993 Apr; 43(4): 662–7
The IFNB Multiple Sclerosis Study Group, University of British Columbia MS/MRI Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology 1995 Jul; 45: 1277–85
Ross C, Clemmesen KM, Svenson M, et al. Immunogenicity of interferon-beta in multiple sclerosis patients: influence of preparation, dosage, dose frequency, and route of administration. Ann Neurol 2000; 48(5): 706–12
Fillipi M, Rocca MA, Camesasca F, et al. IFNB-1b compared with GA therapy for reducing evolution of permanent T1 hypointensities (“black holes”) on brain MRI in treatment-naive RRMS patients [abstract no. PO5.054 plus poster]. 62nd Annual Meeting of the American Academy of Neurology; 2010 Apr 10–17; Toronto
Fillipi M, Rocca MA, Camesasca F, et al. T1-hypointense permanent black holes in RRMS patients treated with interferon beta-1b and glatiramer acetate [abstract no. P-476 plus poster]. 26th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); 2010 Oct 13–16; Gothenburg
Kappos L, Weinshenker B, Pozzilli C, et al. Interferon beta-1b in secondary progressive MS: acombined analysis of the two trials. Neurology 2004 Nov 23; 63(10): 1779–87
Barkhof F, van Waesberghe JH, Filippi M, et al. T(1) hypointense lesions in secondary progressive multiple sclerosis: effect of interferon beta-1b treatment. Brain 2001 Jul; 124 (Pt 7): 1396–402
Brex PA, Molyneux PD, Smiddy P, et al. The effect of IFNbeta-1b on the evolution of enhancing lesions in secondary progressive MS. Neurology 2001 Dec 26; 57(12): 2185–90
Freeman JA, Thompson AJ, Fitzpatrick R, et al. Interferon-beta1b in the treatment of secondary progressive MS: impact on quality of life. Neurology 2001 Nov 27; 57(10): 1870–5
Reder AT, O’Connor P, Kappos L, et al. Short- and long-term safety and tolerability of interferon-beta-1b in multiple sclerosis [abstract no. S105 plus poster]. 24th Annual Meeting of the Consortium of Multiple Sclerosis Centers; 2010 Jun 2–5; San Antonio (TX)
Reder AT, Ebers GC, Traboulsee A, et al. Cross-sectional study assessing long-term safety of interferon-beta-1b for relapsing-remitting MS. Neurology 2010 Jun 8; 74(23): 1877–85
Ebers GC, Reder AT, Traboulsee A, et al. Long-term follow-up of the original interferon-beta1b trial in multiple sclerosis: design and lessons from a 16-year observational study. Clin Ther 2009 Aug; 31(8): 1724–36
Reder AT, Ebers G, Cutter G, et al. Survival analysis 21 years after the initiation of the pivotal interferon beta-1b trial in patients with RRMS [abstract no. P-903 plus poster]. 26th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); 2010 Oct 13–16; Gothenburg
Bell C, Graham J, Earnshaw S, et al. Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on long-term clinical data. J Manag Care Pharm 2007 Apr; 13(3): 245–61
Chilcott J, McCabe C, Tappenden P, et al. Modelling the cost effectiveness of interferon beta and glatiramer acetate in the management of multiple sclerosis. Br Med J 2003; 326(7388): 522–5
Goldberg LD, Edwards NC, Fincher C, et al. Comparing the cost-effectiveness of disease-modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosis. J Manag Care Pharm 2009 Sep; 15(7): 543–55
Nuijten M, Mittendorf T. A health-economic evaluation of disease-modifying drugs for the treatment of relapsingremitting multiple sclerosis from the German societal perspective. Clin Ther 2010 Apr; 32(4): 717–28
Tappenden P, McCabe C, Chilcott J, et al. Cost-effectiveness of disease-modifying therapies in the management of multiple sclerosis for the Medicare population. Value Health 2009; 12(5): 657–65
Touchette DR, Durgin TL, Wanke LA, et al. A cost-utility analysis of mitoxantrone hydrochloride and interferon beta-1b in the treatment of patients with secondary progressive or progressive relapsing multiple sclerosis. Clin Ther 2003 Feb; 25(2): 611–34
National Institute for Clinical Excellence. Beta interferon and glatiramer acetate for the treatment of multiple sclerosis: technology appraisal guidance — no. 32. London: National Institute for Clinical Excellence, 2002
Gani R, Giovannoni G, Bates D, et al. Cost-effectiveness analyses of natalizumab (Tysabri®) compared with other disease-modifying therapies for people with highly active relapsing-remitting multiple sclerosis in the UK. Pharmacoeconomics 2008; 26(7): 617–27
Association of British Neurologists. Revised (2009) guidelines for prescribing in multiple sclerosis [online]. Available from URL: http://www.theabn.org/abn/userfiles/file/ABN_MS_Guidelines_2009_Final.pdf [Accessed 2010 Aug 13]
Compston A, Coles A. Multiple sclerosis. Lancet 2008 Oct 25; 372(9648): 1502–17
Menge T, Weber MS, Hemmer B, et al. Disease-modifying agents for multiple sclerosis: recent advances and future prospects. Drugs 2008; 68(17): 2445–68
Multiple Sclerosis Therapy Consensus Group. Escalating immunotherapy of multiple sclerosis: new aspects and practical application. J Neurol 2004 Nov; 251(11): 1329–39
National Institute for Clinical Excellence. Multiple sclerosis: management of multiple sclerosis in primary and secondary care [online]. Available from URL: http://www.nice.org.uk/nicemedia/pdf/cg008guidance.pdf [Accessed 2010 Aug 13]
Carter NJ, Keating GM. Glatiramer acetate: a review of its use in relapsing-remitting multiple sclerosis and in delaying the onset of clinically definite multiple sclerosis. Drugs 2010; 70(12): 1545–77
Marriott JJ, Miyasaki JN, Gronseth G, et al. Evidence report: the efficacy and safety of mitoxantrone (Novantrone) in the treatment of multiple sclerosis: report of the Therapeutics and Technology Assessment Subcomittee of the American Academy of Neurology. Neurology 2010 May 4; 74(18): 1463–70
Multiple sclerosis drug Gilenya (fingolimod) approved by FDA [online]. Available from URL: http://www.medicalnewstoday.com/printerfriendlynews.php?newsid=20219 [Accessed 2010 Sep 29]
Jeffrey S. European regulators issue negative opinion on cladribine for multiple sclerosis [online]. Available from URL: http://www.medscape.com/viewarticle/729387 [Accessed 2010 Sep 27]
Oral laquinimod for multiple sclerosis granted fast track status by FDA [online]. Available from URL: http://www.medicalnewstoday.com/pritnerfriendlynews.php?newsid=139148 [Accessed 2010 Sep 29]
Carroll WM. Oral therapy for multiple sclerosis: sea change or incremental step? N Engl J Med 2010 Feb 4; 362(5): 456–8
Conway D, Cohen JA. Combination therapy in multiple sclerosis. Lancet Neurol 2010 Mar; 9(3): 299–308
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by:F. Deisenhammer, Department of Neurology, Innsbruck Medical University, Innsbruck, Austria; L. Durelli, Divisione Universitaria Di Neurologia, Facoltà San Luigi Gonzaga, Università Degli Studi Di Torino, Ospedale Clinicizzato San Luigi, Orbassano, Italy; O. Fernandez, Institute of Neurosciences, Service of Neurology, Hospital Regional Universitario Carlos Haya, Malaga, Spain; M.S. Freedman, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Ontario, Canada; A. Traboulsee, Division of Neurology, University of British Columbia, Vancouver, British Columbia, Canada.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘interferon-β-1b’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were (‘interferon beta 1b’ or ‘interferon beta serine’) and ‘multiple sclerosis’. Searches were last updated 19 November 2010.
Selection: Studies in patients with multiple sclerosis who received interferon-β-1b. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Interferon-β-1b, multiple sclerosis, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability, pharmacoeconomics.
Rights and permissions
About this article
Cite this article
Plosker, G.L. Interferon-β-1b. CNS Drugs 25, 67–88 (2011). https://doi.org/10.2165/11206430-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11206430-000000000-00000