Abstract
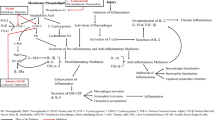
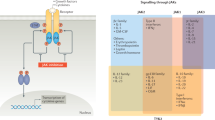
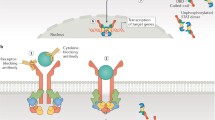
This‘state-of-the-art’ review specifically focuses on alternative signalling pathways deeply involved in acute and chronic inflammatory responses initiated by various pathological stimuli. The accumulated scientific knowledge has already revealed key biological targets, such as COX-2, and related proinflammatory mediators (cytokines and chemokines, interleukins [ILs], tumour necrosis factor [TNF]-α, migration inhibition factor [MIF], interferon [IFN]-ψ and matrix metalloproteinases [MMPs]) implicated in uncontrolled, destructive inflammatory reaction. A number of physiologically active agents are currently approved for market or are under active investigation in different clinical trials. However, recent findings have exposed the fatal adverse effects directly associated with drug therapy based on COX-2 inhibition. Given these possible harmful outcomes, a range of novel therapeutically relevant biological targets that include nuclear transcription factor (NF-κB), p38 mitogen-activated protein kinases (MAPK) and Janus protein tyrosine kinases and signal transducers and activators of transcription (JAK/STAT) signalling pathways has received growing attention. Here we discuss recent progress in the identification and development of novel, clinically approved or evaluated small-molecule regulators of these signalling cascades as promising anti-inflammatory drugs.












Similar content being viewed by others
References
Kulkarni RG, Achaiah G, Sastry GN. Novel targets for antiinflammatory and antiarthritic agents. Curr Pharm Des 2006; 12: 2437–54
Chung HY, Cesari M, Anton S, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 2008. Epub ahead of print
Tincani A, Andreoli L, Bazzani C, et al. Inflammatory molecules: a target for treatment of systemic autoimmune diseases. Autoimmun Rev 2007; 7: 1–7
Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-in-flammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 2006; 332: 1302–8
Silvani MC, Motola D, Poluzzi E, et al. Gastro-intestinal problems and concomitant medication in NSAID users: additional findings from a questionnaire-based survey in Italy. Eur J Clin Pharmacol 2006 Mar; 62 (3): 235–41
Hawkey CJ. Non-steroidal anti-inflammatory drugs: who should receive prophylaxis? Aliment Pharmacol Ther 2004 Jul; 20 Suppl. 2: 59–64
Lamkanfi M, D’hondt K, Vande Walle L, et al. A novel caspase-2 complex containing TRAF2 and RIP1. J Biol Chem 2005; 280: 6923–32
Olah M, Mracec M, Ostopovici L, et al. WOMBAT: world of molecular bioactivity. In: Oprea TI, editor. Cheminformatics in drug discovery. Weinheim: Wiley-VCH, 2004: 223–39
Vanco J. The Beilstein CrossFire Information System and its use in pharmaceutical chemistry. Ceska Slov Farm 2003; 52: 68–72
Baldwin AS Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Ann Rev Immunol 1996; 14: 649–83
Calzado MA, Bacher S, Schmitz ML. NF-kappaB inhibitors for the treatment of inflammatory diseases and cancer. Curr Med Chem 2007; 14 (3): 367–76
Sarkar FH, Li Y, Wang Z, et al. NF-kappaB signaling pathway and its therapeutic implications in human diseases. Int Rev Immunol 2008; 27 (5): 293–319
Cameron NE, Cotter MA. Pro-inflammatory mechanisms in diabetic neuropathy: focus on the nuclear factor kappa B pathway. Curr Drug Targets 2008 Jan; 9 (1): 60–7
Simmonds RE, Foxwell BM. Signaling, inflammation and arthritis: NF-kappaB and its relevance to arthritis and inflammation. Rheumatology 2008 May; 47 (5): 584–90
Basak S, Hoffmann A. Crosstalk via the NF-kappaB signaling system. Cytokine Growth Factor Rev 2008 Jun–Aug; 19 (3–4): 187–97
Uwe S. Anti-inflammatory interventions of NF-kappaB signaling: potential applications and risks. Biochem Pharmacol 2008 Apr; 75 (8): 1567–79
Pomerantz Joel L, Baltimore David. Two pathways to NF-KappaB. Molecular Cell 2002 Oct; 10 (4): 693–5
Oldfield V, Perry CM. Oxycodone/ibuprofen combination tablet: a review of its use in the management of acute pain. Drugs 2005; 65 (16): 2337–54
Palayoor ST, Youmell MY, Calderwood SK, et al. Constitutive activation of IkappaB kinase alpha and NF-kappaB in prostate cancer cells is inhibited by ibuprofen. Oncogene 1999; 18 (51): 389–94
Bachmann AS. Proteasome inhibitors in pediatric cancer treatment. Hawaii Med J 2008 Sep; 67 (9): 247–9
Sinn DI, Lee ST, Chu K, et al. Proteasomal inhibition in intracerebral hemorrhage: neuroprotective and anti-inflammatory effects of bortezomib. Neurosci Res 2007 May; 58 (1): 12–8
Roccaro AM, Hideshima T, Richardson PG, et al. Bortezomib as an antitumor agent. Curr Pharm Biotechnol 2006 Dec; 7 (6): 441–8
Zhang N, Ahsan MH, Zhu L, et al. NF-kappaB and not the MAPK signaling pathway regulates GADD45beta expression during acute inflammation. J Biol Chem 2005 Jun; 280 (22): 21400–8
Steinke JW, Culp JA. Leukotriene synthesis inhibitors versus antagonists: the pros and cons. Curr Allergy Asthma Rep 2007 May; 7 (2): 126–33
Kawano T, Matsuse H, Kondo Y, et al. Cysteinyl leukotrienes induce nuclear factor kappa b activation and RANTES produc-tion in a murine model of asthma. J Allergy Clin Immunol 2003 Aug; 112 (2): 369–74
Lee KS, Kim SR, Park HS, et al. Cysteinyl leukotriene upregulates IL-11 expression in allergic airway disease of mice. J Allergy Clin Immunol 2007 Jan; 119 (1): 141–9
Fukushima C, Matsuse H, Hishikawa Y, et al. Pranlukast, a leukotriene receptor antagonist, inhibits interleukin-5 produc-tion via a mechanism distinct from leukotriene receptor ant-agonism. Int Arch Allergy Immunol 2005 Feb; 136 (2): 165–72
Ishinaga H, Takeuchi K, Kishioka C, et al. Pranlukast inhibits NF-kappaB activation and MUC2 gene expression in cultured human epithelial cells. Pharmacology 2005 Feb; 73 (2): 89–96
Tomari S, Matsuse H, Machida I, et al. Pranlukast, a cysteinyl leukotriene receptor 1 antagonist, attenuates allergen-specific tumour necrosis factor alpha production and nuclear factor kappa B nuclear translocation in peripheral blood monocytes from atopic asthmatics. Clin Exp Allergy 2003 Jun; 33 (6): 795–801
Takahashi H, Funahashi H, Sawai H, et al. Synthetic serine protease inhibitor, gabexate mesilate, prevents nuclear factor-kappaB activation and increases TNF-alpha-mediated apopto-sis in human pancreatic cancer cells. Dig Dis Sci 2007 Oct; 52 (10): 2646–52
Uchiba M, Okajima K, Kaun C, et al. Gabexate mesilate, a synthetic anticoagulant, inhibits the expression of endothelial leukocyte adhesion molecules in vitro. Crit Care Med 2003 Apr; 31 (4): 1147–53
Yuksel M, Okajima K, Uchiba M, et al. Gabexate mesilate, a synthetic protease inhibitor, inhibits lipopolysaccharide-in-duced tumor necrosis factor-alpha production by inhibiting activation of both nuclear factor-kappaB and activator pro-tein-1 in human monocytes. J Pharmacol Exp Ther 2003 Apr; 305 (1): 298–305
Uchiba M, Okajima K, Kaun C, et al. Gabexate mesilate, a synthetic anticoagulant, inhibits the expression of endothelial leukocyte adhesion molecules in vitro. Crit Care Med 2003 Apr; 31 (4): 1284–5
Vandermeeren M, Janssens S, Borgers M, et al. Dimethylfumarate is an inhibitor of cytokine-induced E-selec-tin, VCAM-1, and ICAM-1 expression in human endothelial cells. Biochem Biophys Res Commun 1997 May; 234 (1): 19–23
Loewe R, Holnthoner W, Gröger M, et al. Dimethylfumarate inhibits TNF-induced nuclear entry of NF-kappa B/p65 in human endothelial cells. J Immunol 2002 May; 168 (9): 4781–7
Meili-Butz S, Niermann T, Fasler-Kan E, et al. Dimethyl fumarate, a small molecule drug for psoriasis, inhibits nuclear factor-kappaB and reduces myocardial infarct size in rats. Eur J Pharmacol 2008 May; 586 (1–3): 251–8
Hohensinner PJ, Kaun C, Rychli K, et al. Macrophage colony stimulating factor expression in human cardiac cells is upregu-lated by tumor necrosis factor-alpha via an NF-kappaB dependent mechanism. J Thromb Haemost 2007 Dec; 5 (12): 2520–8
Gesser B, Johansen C, Rasmussen MK, et al. Dimethylfumarate specifically inhibits the mitogen and stress-activated kinases 1 and 2 (MSK1/2): possible role for its anti-psoriatic effect. J Invest Dermatol 2007 Sep; 127 (9): 2129–37
Gerdes S, Shakery K, Mrowietz U. Dimethylfumarate inhibits nuclear binding of nuclear factor kappaB but not of nuclear factor of activated T cells and CCAAT/enhancer binding protein beta in activated human T cells. Br J Dermatol 2007 May; 156 (5): 838–42
Treumer F, Zhu K, Gläser R, et al. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J Invest Dermatol 2003 Dec; 121 (6): 1383–8
Nam NH. Naturally occurring NF-kappaB inhibitors. Mini Rev Med Chem 2006 Aug; 6 (8): 945–51
Clarke JO, Mullin GE. A review of complementary and alternative approaches to immunomodulation. Nutr Clin Pract 2008; 23: 49–62
Jagetia GC, Aggarwal BB. ‘Spicing up’ of the immune system by curcumin. J Clin Immunol 2007 Jan; 27 (1): 19–35
O’Connell MA, Rushworth SA. Curcumin: potential for hepatic fibrosis therapy? Br J Pharmacol 2008 Feb; 153 (3): 403–5
Biswas S, Rahman I. Modulation of steroid activity in chronic inflammation: a novel anti-inflammatory role for curcumin. Mol Nutr Food Res 2008 Mar. Epub ahead of print
Cho JW, Lee KS, Kim CW. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int J Mol Med 2007 Mar; 19 (3): 469–74
Shakibaei M, John T, Schulze-Tanzil G, et al. Suppression of NF-kappaB activation by curcumin leads to inhibition of ex-pression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: implications for the treatment of osteoarthritis. Biochem Pharmacol 2007 May; 73 (9): 1434–45
Jin CY, Lee JD, Park C, et al. Curcumin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated BV2 microglia. Acta Pharmacol Sin 2007 Oct; 28 (10): 1645–51
Sandur SK, Ichikawa H, Pandey MK, et al. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radic Biol Med 2007 Aug; 43 (4): 568–80
Bachmeier BE, Mohrenz IV, Mirisola V, et al. Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB. Carcinogenesis 2008 Apr; 29 (4): 779–89
Kamohara H, Takahashi M, Ishiko T, et al. Induction of interleukin-8 (CXCL-8) by tumor necrosis factor-alpha and leukemia inhibitory factor in pancreatic carcinoma cells: impact of CXCL-8 as an autocrine growth factor. Int J Oncol 2007 Sep; 31 (3): 627–32
Kim YS, Ahn Y, Hong MH, et al. Curcumin attenuates inflammatory responses of TNF-alpha-stimulated human endo-thelial cells. J Cardiovasc Pharmacol 2007 Jul; 50 (1): 41–9
Amoli MM, Mousavizadeh R, Sorouri R, et al. Curcumin inhibits in vitro MCP-1 release from mouse pancreatic islets. Transplant Proc 2006 Nov; 38 (9): 3035–8
Liu YC, Hsieh CW, Wu CC, et al. Chalcone inhibits the activation of NF-kappaB and STAT3 in endothelial cells via endogenous electrophile. Life Sci 2007 Mar; 80 (15): 1420–30
Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 2008 Jul; 149 (7): 3549–58
Mackenzie GG, Queisser N, Wolfson ML, et al. Curcumin induces cell-arrest and apoptosis in association with the inhibition of constitutively active NF-kappaB and STAT3 pathways in Hodgkin’s lymphoma cells. Int J Cancer 2008 Jul; 123 (1): 56–65
Huang S, Zhao L, Kim K, et al. Inhibition of Nod2 signaling and target gene expression by curcumin. Mol Pharmacol 2008 Jul; 74 (1): 274–81
Chen A, Zheng S. Curcumin inhibits connective tissue growth factor gene expression in activated hepatic stellate cells in vitro by blocking NF-kappaB and ERK signaling. Br J Pharmacol 2008 Feb; 153 (3): 557–67
Saja K, Babu MS, Karunagaran D, et al. Anti-inflammatory effect of curcumin involves downregulation of MMP-9 in blood mononuclear cells. Int Immunopharmacol 2007 Dec; 7 (13): 1659–67
Lee CW, Lin CC, Lin WN, et al. TNF-alpha induces MMP-9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF-kappaB/p300 binding in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2007 Mar; 292 (3): L799–812
Choi BH, Kim CG, Lim Y, et al. Curcumin down-regulates the multidrug-resistance mdr1b gene by inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett 2008 Jan; 259 (1): 111–8
Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegen-erative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol 2008. Epub ahead of print
Wadsworth TL, Koop DR. Effects of the wine polyphenolics quercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages. Biochem Pharmacol 1999 Apr; 57 (8): 941–9
Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkap-paB in macrophages by resveratrol. Br J Pharmacol 1999 Feb; 126 (3): 673–80
Holmes-McNary M, Baldwin AS Jr. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res 2000 Jul; 60 (13): 3477–83
Surh YJ, Chun KS, Cha HH, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res 2001 Sep 1; (480–481): 243–68
Pellegatta F, Bertelli AA, Staels B, et al. Different short- and long-term effects of resveratrol on nuclear factor-kappaB phosphorylation and nuclear appearance in human endothelial cells. Am J Clin Nutr 2003 May; 77 (5): 1220–8
Ashikawa K, Majumdar S, Banerjee S, et al. Piceatannol inhibits TNF-induced NF-kappaB activation and NF-kappaB-mediated gene expression through suppression of IkappaBalpha kinase and p65 phosphorylation. J Immunol 2002 Dec; 169 (11): 6490–7
Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol 2000 Jun; 164 (12): 6509–19
Donnelly LE, Newton R, Kennedy GE, et al. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol 2004 Oct; 287 (4): L774–83
Bi XL, Yang JY, Dong YX, et al. Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-acti-vated microglia. Int Immunopharmacol 2005 Jan; 5 (1): 185–93
Meng XL, Chen GL, Yang JY, et al. Inhibitory effect of a novel resveratrol derivative on nitric oxide production in lipo-polysaccharide-activated microglia. Pharmazie 2008 Sep; 63 (9): 671–5
Meng Y, Ma QY, Kou XP, et al. Effect of resveratrol on activation of nuclear factor kappa-B and inflammatory factors in rat model of acute pancreatitis. World J Gastroenterol 2005 Jan; 11 (4): 525–8
Leiro J, Arranz JA, Fraiz N, et al. Effect of cis-resveratrol on genes involved in nuclear factor kappa B signaling. Int Immunopharmacol 2005 Feb; 5 (2): 393–406
de la Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res 2005 May; 49 (5): 405–30
Elmali N, Baysal O, Harma A, et al. Effects of resveratrol in inflammatory arthritis. Inflammation 2007 Apr; 30 (1–2): 1–6
Birrell MA, McCluskie K, Wong S, et al. Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-kappaB-independent mechanism. FASEB J 2005 May; 19 (7): 840–1
Youn HS, Lee JY, Fitzgerald KA, et al. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: molecular targets are TBK1 and RIP1 in TRIF complex. J Immunol 2005 Sep; 175 (5): 3339–46
Zhu J, Yong W, Wu X, et al. Anti-inflammatory effect of resveratrol on TNF-alpha-induced MCP-1 expression in adipocytes. Biochem Biophys Res Commun 2008 May; 369 (2): 471–7
Kishore N, Sommers C, Mathialagan S, et al. A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J Biol Chem 2003; 278: 32861–71
Phulwani NK, Esen N, Syed MM, et al. TLR2 expression in astrocytes is induced by TNF-alpha- and NF-kappaB-depen-dent pathways. J Immunol 2008; 181: 3841–9
Antunes TT, Gagnon A, Langille ML, et al. Thyroid-stimulating hormone induces interleukin-6 release from human adipocytes through activation of the nuclear factor-kappaB pathway. Endocrinology 2008; 149: 3062–6
Choo MK, Sakurai H, Kim DH, et al. A ginseng saponin metabolite suppresses tumor necrosis factor-alpha-promoted metastasis by suppressing nuclear factor-kappaB signaling in murine colon cancer cells. Oncol Rep 2008; 19: 595–600
Hwang DM, Kundu JK, Shin JW, et al. Cis-9,trans-11-conjugated linoleic acid down-regulates phorbol ester-induced NF-kappaB activation and subsequent COX-2 expression in hairless mouse skin by targeting IkappaB kinase and PI3K-Akt. Carcinogenesis 2007; 28: 363–71
Killeen ME, Englert JA, Stolz DB, et al. The phase 2 enzyme inducers ethacrynic acid, DL-sulforaphane, and oltipraz inhibit lipopolysaccharide-induced high-mobility group box 1 secretion by RAW 264.7 cells. J. Pharmacol Exp Ther 2006; 316: 1070–9
Gomez AB, MacKenzie C, Paul A, et al. Selective inhibition of inhibitory kappa B kinase-beta abrogates induction of nitric oxide synthase in lipopolysaccharide-stimulated rat aortic smooth muscle cells. Br J Pharmacol 2005; 146: 217–25
Peng Y, Power MR, Li B, et al. Inhibition of IKK down-regulates antigen + IgE-induced TNF production by mast cells: a role for the IKK-IkappaB-NF-kappaB pathway in IgE-de-pendent mast cell activation. J Leukoc Biol 2005; 77: 975–83
Podolin PL, Callahan JF, Bolognese BJ, et al. Attenuation of murine collagen-induced arthritis by a novel, potent, selective small molecule inhibitor of IkappaB kinase 2, TPCA-1 (2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophene-carboxamide), occurs via reduction of proinflammatory cyto-kines and antigen-induced T cell proliferation. J Pharmacol Exp Ther 2005; 312: 373–81
Kondo Y, Fukuda K, Adachi T, et al. Inhibition by a selective I{kappa}B kinase-2 inhibitor of interleukin-1-induced collagen degradation by corneal fibroblasts in three-dimensional culture. Invest Ophthalmol Vis Sci 2008. Epub ahead of print
Tudhope SJ, Catley MC, Fenwick PS, et al. The role of IkappaB kinase 2, but not activation of NF-kappaB, in the release of CXCR3 ligands from IFN-gamma-stimulated human bronchial epithelial cells. J Immunol 2007; 179: 6237–45
Birrell MA, Wong S, Hardaker EL, et al. IkappaB kinase-2-independent and -dependent inflammation in airway disease models: relevance of IKK-2 inhibition to the clinic. Mol Pharmacol 2006; 69: 1791–800
Birrell MA, McCluskie K, Hardaker E, et al. Utility of exhaled nitric oxide as a noninvasive biomarker of lung inflammation in a disease model. Eur Respir J 2006; 28: 1236–44
Beaulieu F, Ouellet C, Ruediger EH, et al. Synthesis and biological evaluation of 4-amino derivatives of benzimidazoquinoxaline, benzimidazoquinoline, and benzopyrazoloquinazoline as potent IKK inhibitors. Bioorg Med Chem Lett 2007; 17: 1233–7
Burke JR, Pattoli MA, Gregor KR, et al. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem 2003; 278: 1450–6
Scott T, Owens MD. Thrombocytes respond to lipopolysaccharide through Toll-like receptor-4, and MAP kinase and NF-kappaB pathways leading to expression of interleukin-6 and cyclooxygenase-2 with production of prostaglandin E2. Mol Immunol 2008; 45: 1001–8
McIntyre KW, Shuster DJ, Gillooly KM, et al. A highly selective inhibitor of I kappa B kinase, BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in mice. Arthritis Rheum 2003 Sep; 48 (9): 2652–9
Townsend RM, Postelnek J, Susulic V, et al. A highly selective inhibitor of IkappaB kinase, BMS-345541, augments graft survival mediated by suboptimal immunosuppression in a murine model of cardiac graft rejection. Transplantation 2004; 77: 1090–4
Bianchi G, Montecucco F, Bertolotto M, et al. Immune complexes induce monocyte survival through defined intracellular pathways. Ann N Y Acad Sci 2007 Jan; 1095: 209–19
Karehed K, Dimberg A, Dahl S, et al. IFN-gamma-induced upregulation of Fcgamma-receptor-I during activation of monocytic cells requires the PKR and NFkappaB pathways. Mol Immunol 2007; 44: 615–24
Katdare M, Efimova EV, Labay E, et al. Diverse TNFalpha-induced death pathways are enhanced by inhibition of NF-kappaB. Int J Oncol 2007; 31: 1519–28
Keslacy S, Tliba O, Baidouri H, et al. Inhibition of tumor necrosis factor-alpha-inducible inflammatory genes by inter-feron-gamma is associated with altered nuclear factor-kappaB transactivation and enhanced histone deacetylase activity. Mol Pharmacol 2007 Feb; 71 (2): 609–18
Keslacy S, Tliba O, Baidouri H, et al. Inhibition of tumor necrosis factor-alpha-inducible inflammatory genes by interferon-gamma is associated with altered nuclear factor-kappaB transactivation and enhanced histone deacetylase activity. Mol Pharmacol 2007; 71: 609–18
Rhee JW, Lee KW, Sohn WJ, et al. Regulation of matrix metalloproteinase-9 gene expression and cell migration by NF-kappa B in response to CpG-oligodeoxynucleotides in RAW 264.7 cells. Mol Immunol 2007; 44: 1393–400
Pattoli MA, MacMaster JF, Gregor KR, et al. Collagen and aggrecan degradation is blocked in interleukin-1-treated cartilage explants by an inhibitor of IkappaB kinase through suppression of metalloproteinase expression. J Pharmacol Exp Ther 2005; 315: 382–8
Everhart MB, Han W, Sherrill TP, et al. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J Immunol 2006; 176: 4995–5005
MacMaster JF, Dambach DM, Lee DB, et al. An inhibitor of IkappaB kinase, BMS-345541, blocks endothelial cell adhesion molecule expression and reduces the severity of dextran sulfate sodium-induced colitis in mice. Inflamm Res 2003; 52: 508–11
McIntyre KW, Shuster DJ, Gillooly KM, et al. A highly selective inhibitor of I kappa B kinase, BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in mice. Arthritis Rheum 2003; 48: 2652–9
Schopf L, Savinainen A, Anderson K, et al. IKKbeta inhibition protects against bone and cartilage destruction in a rat model of rheumatoid arthritis. Arthritis Rheum 2006; 54: 3163–73
Wen D, Nong Y, Morgan JG, et al. A selective small molecule IkappaB kinase beta inhibitor blocks nuclear factor kappaB-mediated inflammatory responses in human fibroblast-like synoviocytes, chondrocytes, and mast cells. J Pharmacol Exp Ther 2006; 317: 989–1001
Schopf L, Savinainen A, Anderson K, et al. IKKbeta inhibition protects against bone and cartilage destruction in a rat model of rheumatoid arthritis. Arthritis Rheum 2006 Oct; 54 (10): 3163–73
Izmailova ES, Paz N, Alencar H, et al. Use of molecular imaging to quantify response to IKK-2 inhibitor treatment in murine arthritis. Arthritis Rheum 2007; 56: 117–28
Nagashima K, Sasseville VG, Wen D, et al. Rapid TNFR1-dependent lymphocyte depletion in vivo with a selective chemical inhibitor of IKKbeta. Blood 2006; 107: 4266–73
Newton R, Holden NS, Catley MC, et al. Repression of inflammatory gene expression in human pulmonary epithelial cells by small-molecule IkappaB kinase inhibitors. J Pharmacol Exp Ther 2007; 321: 734–42
Catley MC, Sukkar MB, Chung KF, et al. Validation of the antiinflammatory properties of small-molecule IkappaB Kinase (IKK)-2 inhibitors by comparison with adenoviral-mediated delivery of dominant-negative IKK1 and IKK2 in human airways smooth muscle. Mol Pharmacol 2006; 70: 697–705
El-Remessy AB, Al-Shabrawey M, Khalifa Y, et al. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol 2006 Jan; 168 (1): 235–44
Esposito G, De Filippis D, Maiuri MC, et al. Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in beta-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-kappaB involvement. Neurosci Lett 2006 May; 399 (1–2): 91–5
McKallip RJ, Jia W, Schlomer J, et al. Cannabidiol-induced apoptosis in human leukemia cells: a novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol Pharmacol 2006 Sep; 70 (3): 897–908
Chen J, Errico SL, Freed WJ. Reactive oxygen species and p38 phosphorylation regulate the protective effect of delta9-tetrahydrocannabinol in the apoptotic response to NMDA. Neurosci Lett 2005 Dec; 389 (2): 99–103
Simi A, Porsmyr-Palmertz M, Hjertén A, et al. The neuroprotective agents chlomethiazole and SB203580 inhibit IL-1beta signaling but not its biosynthesis in rat cortical glial cells. J Neurochem 2002 Nov; 83 (3): 727–37
Simi A, Ingelman-Sundberg M, Tindberg N. Neuroprotective agent chlomethiazole attenuates c-fos, c-jun, and AP-1 activation through inhibition of p38 MAP kinase. J Cereb Blood Flow Metab 2000 Jul; 20 (7): 1077–88
Tindberg N, Baldwin HA, Cross AJ, et al. Induction of cytochrome P450 2E1 expression in rat and gerbil astrocytes by inflammatory factors and ischemic injury. Mol Pharmacol 1996 Nov; 50 (5): 1065–72
Trompezinski S, Migdal C, Tailhardat M, et al. Characterization of early events involved in human dendritic cell maturation induced by sensitizers: cross talk between MAPK signaling pathways. Toxicol Appl Pharmacol 2008 Apr. Epub ahead of print
Dai X, Sayama K, Tohyama M, et al. The NF-{kappa} B, p38 MAPK and STAT1 pathways differentially regulate the dsRNA-mediated innate immune responses of epidermal keratinocytes. Int Immunol 2008 Jul; 20 (7): 901–9
Wang X, Xue H, Xu Q, et al. p38 kinase/cytosolic phospholipase A(2)/cyclooxygenase-2 pathway: a new signaling cascade for lipopolysaccharide-induced interleukin-1beta and interleukin-6 release in differentiated U937 cells. Prostaglandins Other Lipid Mediat 2008 Jun; 86 (1–4): 61–7
von Brandenstein MG, Ngum Abety A, Depping R, et al. A p38–p65 transcription complex induced by endothelin-1 mediates signal transduction in cancer cells. Biochim Biophys Acta 2008 Sep; 1783 (9): 1613–22
Liu S, Feng G, Wang GL, et al. p38MAPK inhibition attenuates LPS-induced acute lung injury involvement of NF-kappaB pathway. Eur J Pharmacol 2008 Apr 14; 584 (1): 159–65
Lee JC, Yu MK, Lee R, et al. Terrein reduces pulpal inflammation in human dental pulp cells. J Endod 2008 Apr; 34 (4): 433–7
Ulivi V, Giannoni P, Gentili C, et al. p38/NF-kB-dependent expression of COX-2 during differentiation and inflammatory response of chondrocytes. J Cell Biochem 2008 Jul; 104 (4): 1393–406
Hsu JT, Kan WH, Hsieh CH, et al. Mechanism of estrogen-mediated intestinal protection following trauma-hemorrhage: p38 MAPK-dependent upregulation of HO-1. Am J Physiol Regul Integr Comp Physiol 2008 Jun; 294 (6): R1825–31
Méndez-Samperio P, Trejo A, Pérez A. Mycobacterium bovis Bacillus Calmette-Guérin (BCG) stimulates IL-10 production via the PI3K/Akt and p38 MAPK pathways in human lung epithelial cells. Cell Immunol 2008 Jan; 251 (1): 37–42
Chaparro-Huerta V, Flores-Soto ME, Gudiño-Cabrera G, et al. Role of p38 MAPK and pro-inflammatory cytokines expression in glutamate-induced neuronal death of neonatal rats. Int J Dev Neurosci 2008 Aug; 26 (5): 487–95
Hisatsune J, Nakayama M, Isomoto H, et al. Molecular characterization of Helicobacter pylori VacA induction of IL-8 in U937 cells reveals a prominent role for p38MAPK in activating transcription factor-2, cAMP response element binding protein, and NF-kappaB activation. J Immunol 2008 Apr; 180 (7): 5017–27
Hayashi S, Jibiki I, Asai Y, et al. Analysis of gene expression in human bronchial epithelial cells upon influenza virus infection and regulation by p38 mitogen-activated protein kinase and c-Jun-N-terminal kinase. Respirology 2008 Mar; 13 (2): 203–14
Rossa C Jr, Liu M, Kirkwood KL. A dominant function of p38 mitogen-activated protein kinase signaling in receptor activator of nuclear factor-kappaB ligand expression and osteoclastogenesis induction by Aggregatibacter actinomycetemcomitans and Escherichia coli lipopolysaccharide. J Periodontal Res 2008 Apr; 43 (2): 201–11
Liu FQ, Liu Y, Lui VC, et al. Hypoxia modulates lipopolysaccharide induced TNF-alpha expression in murine macrophages. Exp Cell Res 2008 Apr; 314 (6): 1327–36
Nishikawa T, Hagihara K, Serada S, et al. Transcriptional complex formation of c-fos, STAT3, and hepatocyte NF-1{alpha} is essential for cytokine-driven C-reactive protein gene expression. J Immunol 2008 Mar; 180 (5): 3492–501
Muniyappa H, Das KC. Activation of c-Jun N-terminal kinase (JNK) by widely used specific p38 MAPK inhibitors SB202190 and SB203580: a MLK-3-MKK7-dependent mechanism. Cell Signal 2008 Apr; 20 (4): 675–83
Muniyappa H, Das KC. Activation of c-Jun N-terminal kinase (JNK) by widely used specific p38 MAPK inhibitors SB202190 and SB203580: a MLK-3-MKK7-dependent mechanism. Cell Signal 2008 Apr; 20 (4): 675–83
You X, Wu Y, Zeng Y, et al. Mycoplasma genitalium-derived lipid-associated membrane proteins induce activation of MAPKs, NF-kappaB and AP-1 in THP-1 cells. FEMS Immunol Med Microbiol 2008 Mar; 52 (2): 228–36
Kuma Y, Sabio G, Bain J, et al. BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo. J Biol Chem 2005; 280: 19472–9
Moss N, Breitfelder S, Betageri R, et al. New modifications to the area of pyrazole-naphthyl urea based p38 MAP kinase inhibitors that bind to the adenine/ATP site. Bioorg Med Chem Lett 2007; 17: 4242–7
Lee MR, Dominguez C. MAP kinase p38 inhibitors: clinical results and an intimate look at their interactions with p38alpha protein. Curr Med Chem 2005; 12: 2979–94
Branger J, van den Blink B, Weijer S, et al. Anti-inflammatory effects of a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. J Immunol 2002; 168: 4070–7
van den Blink B, Branger J, Weijer S, et al. P38 mitogen activated protein kinase is involved in the downregulation of granulocyte CXC chemokine receptors 1 and 2 during human endotoxemia. J Clin Immunol 2004; 24: 37–41
Frembgen-Kesner T, Elcock AH. Computational sampling of a cryptic drug binding site in a protein receptor: explicit solvent molecular dynamics and inhibitor docking to p38 MAP kinase. J Mol Biol 2006; 359: 202–14
Angell RM, Angell TD, Bamborough P, et al. Biphenyl amide p38 kinase inhibitors 4: DFG-in and DFG-out binding modes. Bioorg Med Chem Lett 2008; 18: 4433–7
Sullivan JE, Holdgate GA, Campbell D, et al. Prevention of MKK6-dependent activation by binding to p38alpha MAP kinase. Biochemistry 2005; 44: 16475–90
Ding C. Drug evaluation: VX-702, a MAP kinase inhibitor for rheumatoid arthritis and acute coronary syndrome. Curr Opin Investig Drugs 2006; 7: 1020–5
Kuliopulos A, Mohanlal R, Covic L. Effect of selective inhibition of the p38 MAP kinase pathway on platelet aggregation. Thromb Haemost 2004; 92: 1387–93
Collis AJ, Foster ML, Halley F, et al. RPR-203494 a pyrimidine analogue of the p38 inhibitor RPR200765A with an improved in vitro potency. Bioorg Med Chem Lett 2001; 11: 693–6
Mclay LM, Halley F, Souness JE, et al. The discovery of RPR-200765A, a p38 MAP kinase inhibitor displaying a good oral anti-arthritic efficacy. Bioorg Med Chem 2001; 9: 537–54
Goldstein DM, Alfredson T, Bertrand J, et al. Discovery of S-[5-amino-1-(4-fluorophenyl)-1H-pyrazol-4-yl]-[3-(2,3-dihydroxypropoxy)phenyl]methanone (RO3201195), an orally bioavailable and highly selective inhibitor of p38 MAP kinase. J Med Chem 2006; 49: 1562–75
Ward KW, Proksch JW, Salyers KL, et al. SB-242235, a selective inhibitor of p38 mitogen-activated protein kinase. I: preclinical pharmacokinetics. Xenobiotica 2002; 32: 221–33
Ward KW, Proksch JW, Gorycki PD, et al. SB-242235, a selective inhibitor of p38 mitogen-activated protein kinase. II: in vitro and in vivo metabolism studies and pharmacokinetic extrapolation to man. Xenobiotica 2002; 32: 235–50
Badger AM, Griswold DE, Kapadia R, et al. Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis. Arthritis Rheum 2000; 43: 175–83
Pei Y, Harvey A, Yu XP, et al. Differential regulation of cytokine-induced MMP-1 and MMP-13 expression by p38 kinase inhibitors in human chondrosarcoma cells: potential role of Runx2 in mediating p38 effects. Osteoarthritis Cartilage 2006; 14: 749–58
Patten C, Bush K, Rioja I, et al. Characterization of pristane-induced arthritis, a murine model of chronic disease: response to antirheumatic agents, expression of joint cytokines, and immunopathology. Arthritis Rheum 2004; 50: 3334–45
Kim AL, Labasi JM, Zhu Y, et al. Role of p38 MAPK in UVB-induced inflammatory responses in the skin of SKH-1 hairless mice. J Invest Dermatol 2005; 124: 1318–25
Wadsworth SA, Cavender DE, Beers SA, et al. RWJ 67657, a potent, orally active inhibitor of p38 mitogen-activated protein kinase. J Pharmacol Exp Ther 1999; 291: 680–7
Wadsworth SA, Cavender DE, Beers SA, et al. RWJ 67657, a potent, orally active inhibitor of p38 mitogen-activated protein kinase. J Pharmacol Exp Ther 1999 Nov; 291 (2): 680–7
Westra J, Doornbos-van der Meer B, de Boer P, et al. Strong inhibition of TNF-alpha production and inhibition of IL-8 and COX-2 mRNA expression in monocyte-derived macrophages by RWJ 67657, a p38 mitogen-activated protein kinase (MAPK) inhibitor. Arthritis Res Ther 2004; 6: R384–92
Westra J, Limburg PC, de Boer P, et al. Effects of RWJ 67657, a p38 mitogen activated protein kinase (MAPK) inhibitor, on the production of inflammatory mediators by rheumatoid synovial fibroblasts. Ann Rheum Dis 2004; 63: 1453–9
Fijen JW, Zijlstra JG, De Boer P, et al. Suppression of the clinical and cytokine response to endotoxin by RWJ-67657, a p38 mitogen-activated protein-kinase inhibitor, in healthy human volunteers. Clin Exp Immunol 2001; 124: 16–20
Thurmond RL, Wadsworth SA, Schafer PH, et al. Kinetics of small molecule inhibitor binding to p38 kinase. Eur J Biochem 2001; 268: 5747–54
Parasrampuria DA, de Boer P, Desai-Krieger D, et al. Single-dose pharmacokinetics and pharmacodynamics of RWJ 67657, a specific p38 mitogen-activated protein kinase inhibitor: a first-in-human study. J Clin Pharmacol 2003; 43: 406–13
Faas MM, Moes H, Fijen JW, et al. Monocyte intracellular cytokine production during human endotoxaemia with or without a second in vitro LPS challenge: effect of RWJ-67657, a p38 MAP-kinase inhibitor, on LPS-hyporesponsiveness. Clin Exp Immunol 2002; 127: 337–43
Fijen JW, Tulleken JE, Kobold AC, et al. Inhibition of p38 mitogen-activated protein kinase: dose-dependent suppression of leukocyte and endothelial response after endotoxin challenge in humans. Crit Care Med 2002; 30: 841–5
Westra J, Ku/tdo JM, van Rijswijk MH, et al. Chemokine production and E-selectin expression in activated endothelial cells are inhibited by p38 MAPK (mitogen activated protein kinase) inhibitor RWJ 67657. Int Immunopharmacol 2005; 5: 1259–69
Donnelly LE, Rogers DF. Therapy for chronic obstructive pulmonary disease in the 21st century. Drugs 2003; 63: 1973–98
Lub-de Hooge MN, de Jong S, Vermot-Desroches C, et al. Endotoxin increases plasma soluble tumor necrosis factor-related apoptosis-inducing ligand level mediated by the p38 mitogen-activated protein kinase signaling pathway. Shock 2004; 22: 186–8
Nikas SN, Drosos AA. SCIO-469 Scios Inc. Curr Opin Investig Drugs 2004; 5: 1205–12
Lee MR, Dominguez C. MAP kinase p38 inhibitors: clinical results and an intimate look at their interactions with p38alpha protein. Curr Med Chem 2005; 12: 2979–94
Dominguez C, Powers DA, Tamayo N. p38 MAP kinase inhibitors: many are made, but few are chosen. Curr Opin Drug Discov Devel 2005; 8: 421–30
Ihle J. Pathways in cytokine regulation of hematopoiesis. Ann NY Acad Sci 2001 Jun; 938: 129–30
O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity 2008 Apr; 28 (4): 477–87
Walker JG, Smith MD. The JAK-STAT pathway in rheumatoid arthritis. J Rheumatol 2005 Sep; 32 (9): 1650–3
Kagami S, Saeki H, Komine M, et al. Interleukin-4 and interleukin-13 enhance CCL26 production in a human keratinocyte cell line, HaCaT cells. Clin Exp Immunol 2005 Sep; 141 (3): 459–66
Si HF, Li J, Lü XW, et al. Suppressive effects of leflunomide on leptin-induced collagen I production involved in hepatic stellate cell proliferation. Exp Biol Med 2007 Mar; 232 (3): 427–36
Giles JV, Bergstrom DA, Garcia-Manero G, et al. MK-0457 is a novel Aurora kinase and janus kinase 2 inhibitor with activity in transformed JAK2-positive myeloproliferative disease. 12th Congress of the European Hematology Association; 2007 June 7–10; Vienna, Aust
Hexner EO, Serdikoff C, Jan M, et al. Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood 2008 June; 111 (12): 5663–71
Kudlacz E, Conklyn M, Andresen C, et al. The JAK-3 inhibitor CP-690550 is a potent anti-inflammatory agent in a murine model of pulmonary eosinophilia. Eur J Pharmacol 2008 Mar; 582 (1–3): 154–61
Hood J, Cao C, Chow J, et al. Development of TG101348 for the treatment of JAK2-driven malignancies. J Clin Oncol 2008; 26 (15S): 7083.
Hood J, Doukas M, Martin G, et al. TG101348, a potent, highly selective JAK2 inhibitor, inhibits colony formation in stem cells from polycythemia vera patients and prevents JAK2V617F-mediated splenomegaly and death in a mouse model. J Clin Oncol 2007; 25 (18S): 7031
Kudlacz E, Perry B, Sawyer P, et al. The novel JAK-3 inhibitor CP-690550 is a potent immunosuppressive agent in various murine models. Am J Transplant 2004; 4: 51–7
Borie DC, Si MS, Morris RE, et al. JAK3 inhibition as a new concept for immune suppression. Curr Opin Investig Drugs 2003; 4: 1297–303
Hood J. New drug compounds and future drugs for the treatment and prevention of cancer. 5th Cancer Drugs Research & Development; 2008 February 21–22; Phoenix, AZ
Hood J, Cao J, Chow C, et al. Development of TG101348 for the treatment of JAK2-driven malignancies. J Clin Oncol 2008; 26: 15S, 7083
Hood J, Doukas J, Martin M, et al. TG101348, a potent, highly selective JAK2 inhibitor, inhibits colony formation in stem cells from polycythemia vera patients and prevents JAK2V617F-mediated splenomegaly and death in a mouse model. J Clin Oncol 2007; 25: 18S, 7031
Wernig G, Kharas MG, Okabe R, et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell 2008 Apr; 13 (4): 311–20
Geron I, Abrahamsson AE, Barroga CF, et al. Selective inhibition of JAK2-driven erythroid differentiation of polycythemia vera progenitors. Cancer Cell 2008 Apr; 13 (4): 321–30
Golas JM, Arndt K, Etienne C, et al. SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xeno-grafts in nude mice. Cancer Research 2003 Jan; 63: 375–81
Chai SK, Nichols GL, Rothman P. Constitutive activation of JAKs and STATs in BCR-Abl-expressing cell lines and peripheral blood cells derived from leukemic patients. J Immunol 1997 Nov; 159 (10): 4720–8
Donato N, Wu J, Zhang L, et al. Down-regulation of interleukin-3/granulocyte-macrophage colony-stimulating factor receptor β-chain in Bcr-Abl(+) human leukemic cells: association with loss of cytokine-mediated STAT5 activation and protection from apoptosis after Bcr-Abl inhibition. Blood 2001 May 1; 97 (9): 2846–53
Amit-Vazina M, Shishodia S, Harris D, et al. Atiprimod blocks STAT3 phosphorylation and induces apoptosis in multiple myeloma cells. Br J Cancer 2005 Jul; 93 (1): 70–80
Faderl S, Ferrajoli A, Harris D, et al. Atiprimod blocks phosphorylation of JAK-STAT and inhibits proliferation of acute myeloid leukemia (AML) cells. Leuk Res 2007 Jan; 31 (1): 91–5
Zhou J, Khng J, Jasinghe VJ, et al. In vivo activity of ABT-869, a multi-target kinase inhibitor, against acute myeloid leukemia with wild-type FLT3 receptor. Leuk Res 2008 Jul; 32 (7): 1091–100
Shankar DB, Li J, Tapang P, et al. ABT-869, a multitargeted receptor tyrosine kinase inhibitor: inhibition of FLT3 phospho-rylation and signaling in acute myeloid leukemia. Blood 2007 Apr; 109 (8): 3400–8
Zhou J, Pan M, Xie Z, et al. Synergistic antileukemic effects between ABT-869 and chemotherapy involve downregulation of cell cycle-regulated genes and c-Mos-mediated MAPK pathway. Leukemia 2008 Jan; 22 (1): 138–46
Guo J, Marcotte PA, McCall JO, et al. Inhibition of phosphorylation of the colony-stimulating factor-1 receptor (c-Fms) tyro-sine kinase in transfected cells by ABT-869 and other tyrosine kinase inhibitors. Mol Cancer Ther 2006 Apr; 5 (4): 1007–13
Wei S, Dai XM, Stanley ER. Transgenic expression of CSF-1 in CSF-1 receptor-expressing cells leads to macrophage activation, osteoporosis, and early death. Leukoc Biol 2006 Dec; 80 (6): 1445–53
Acknowledgements
No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ivanenkov, Y.A., Balakin, K.V. & Tkachenko, S.E. New Approaches to the Treatment of Inflammatory Disease. Drugs in R D 9, 397–434 (2008). https://doi.org/10.2165/0126839-200809060-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/0126839-200809060-00005