Abstract
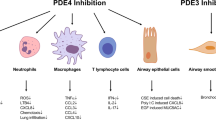
Asthma is a chronic inflammatory condition characterised by reversible airflow obstruction and airway hyperreactivity. The course of the illness may be punctuated by exacerbations resulting in deterioration in quality of life and, in some cases, days lost from school or work. That asthma is common and increasingly prevalent magnifies the importance of any potential economic costs, and promoting asthma control represents an important public health agenda. While lifestyle changes represent a valuable contribution in some patients, the majority of asthmatic patients require therapeutic intervention. The recognition of the role of inflammation in the pathogenesis of asthma has led to an emphasis on regular anti-inflammatory therapy, of which inhaled corticosteroid treatment remains the most superior. In selected patients, further improvements in asthma control may be gained by the addition of regular inhaled long-acting β2-adrenoceptor agonists or oral leukotriene receptor antagonists to inhaled corticosteroid therapy. However, a significant minority of patients with asthma remain poorly controlled despite appropriate treatment, suggesting that additional corticosteroid nonresponsive inflammatory pathways may be operative. Furthermore, some patients with asthma display an accelerated decline in lung function, suggesting that active airway re-modeling is occurring. Such observations have focused attention on the potential to develop new therapies which complement existing treatments by targeting additional inflammatory pathways. The central role of phosphodiesterase (PDE), and in particular the PDE4 enzyme, in the regulation of key inflammatory cells believed to be important in asthma — including eosinophils, lymphocytes, neutrophils and airway smooth muscle — suggests that drugs designed to target this enzyme will have the potential to deliver both bronchodilation and modulate the asthmatic inflammatory response. In vivo studies on individual inflammatory cells suggest that the effects are likely to be favorable in asthma, and animal study models have provided proof of concept; however, first-generation PDE inhibitors have been poorly tolerated due to adverse effects. The development of second-generation agents such as cilomilast and roflumilast heralds a further opportunity to test the potential of these agents, although to date only a limited amount of data from human studies has been published, making it difficult to draw firm conclusions.
Similar content being viewed by others
References
Global initiative for asthma [online]. Available from URL: www.ginasthma.com [Accessed 2005 Jan]
Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy 2004; 59(5): 469–78
British Guideline on the Management of Asthma. Scottish Intercollegiate Guidance Network (SIGN) Guideline 63 [online]. Available from URL: www.sign.ac.uk/guidelines/fulltext/63/index.html [Accessed 2005 Jan]
Lemiere C, Bai T, Baiter M, et al. Adult asthma consensus guidelines update 2003. Can Respir J. 2004; 11 Suppl. A: 9A–18A
Jenkins C. An update on asthma management. Intern Med J 2003; 33: 365–71
Barnes PJ. Inhaled glucocorticoids for asthma. N Engl J Med 1995; 332: 868–75
Djukanovic R, Wilson JW, Britten KM, et al. Effect of an inhaled corticosteroid on airway inflammation and symptoms in asthma. Am Rev Respir Dis 1992; 145: 669–74
Haahtela T, Jarvinen M, Kava T, et al. Comparison of a beta 2-agonist, terbutaline, with an inhaled corticosteroid, budesonide, in newly detected asthma. N Engl J Med 1991; 325: 388–92
Pauwels RA, Pedersen S, Busse WW, et al. Early intervention with budesonide in mild asthma: a randomised, double-blind trial. Lancet 2003; 361: 1071–6
Donahue JG, Weiss ST, Livingston JM, et al. Inhaled steroids and the risk of hospitalisation for asthma. JAMA 1997; 277: 887–91
Eisner MD, Lieu TA, Chi F, et al. Beta agonists, inhaled corticosteroids, and the risk of intensive care unit admission for asthma. Eur Respir J 2002; 17: 233–40
Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000; 343: 332–6
Szefler SJ, Martin RJ, Sharp King T, et al. for the Asthma Clinical Research Network of the National Heart, Lung and Blood Institute.Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol 2002; 109: 410–8
Martin RJ, Szefler SJ, Chinchilli VM, et al. Systemic effect comparisons of six inhaled corticosteroid preparations. Am J Respir Crit Care Med 2002; 165: 1377–83
Bjermer L, Bisgaard H, Bousquet J, et al. Montelukast and fluticasone compared with salmeterol and fluticasone in protecting against asthma exacerbation in adults: one year, double blind, randomised comparative trial. BMJ 2003; 327: 891–5
Greening AP, Ind PW, Northfield M, et al. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Lancet 1994; 344: 219–24
Pauwels RA, Löfdahl C-G, Postma DS, et al. Effect of inhaled formoterol and budesonide in exacerbations of asthma. N Engl J Med 1997; 337: 1405–11
Shewsbury S, Pyke S, Britton M. Meta-analysis of increased dose of inhaled steroid or addition of salmeterol in symptomatic asthma (MIASMA). BMJ 2000; 320: 1368–73
O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma. Am J Respir Crit Care Med 2001; 164: 1392–7
Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma Control study. Am J Respir Crit Care Med 2004; 170: 836–44
Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting β2-agonists and corticosteroids. Eur Respir J 2002; 19: 182–91
Bjermer L, Bisgaard H, Bousquet J, et al. Montelukast and fluticasone compared with salmeterol and fluticasone in protecting against asthma exacerbation in adults: one year, double blind, randomised comparative trial. BMJ 2003; 327: 891–5
Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double-blind placebo-controlled trial. Lancet 2001; 357: 2007–11
Roorda RJ, Gerritsen J, van Aalderen WM, et al. Follow-up of asthma from childhood to adulthood: influence of potential childhood risk factors on the outcome of pulmonary function and bronchial responsiveness in adulthood. J Allergy Clin Immunology 1994; 93: 575–84
Chung KF, Godard P. ERS task force: difficult therapy-resistant asthma. Eur Respir J 1999; 13: 1198–208
Wenzel SE, Szefler SJ, Leung DY, et al. Bronchoscopic evaluation of severe asthma: persistent inflammation associated with high dose corticosteroids. Am J Respir Crit Care Med 1997; 156: 737–43
Payne DN, Adcock IM, Wilson NM, et al. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma after treatment with oral prednisolone. Am J Respir Crit Care Med 2001; 164: 1376–81
The ENFUMOSA cross-sectional multicentre study of the clinical phenotype of chronic severe asthma. European network for understanding mechanisms of severe asthma. Eur Respir J 2003; 22: 470–7
Becker AB, Simons KJ, Gillespie CA, et al. The bronchodilator effects and pharmacokinetics of caffeine in asthma. N Engl J Med 1984; 310: 743–6
Weinberger M, Hendeles L. Theophylline in asthma. N Engl J Med 1996; 334: 1380–8
Magnussen H, Reuss G, Jorres R. Theophylline has a dose-related effect on the airway response to inhaled histamine and methacholine in asthmatics. Am Rev Respir Dis 1987; 136: 1163–7
Magnussen H, Reuss G, Jorres R. Methylxanthines inhibit exercise-induced bronchoconstriction at low serum theophylline concentration and in a dose dependant fashion. J Allergy Clin Immunol 1988; 81: 531–7
Ward AJ, McKenniff M, Evans JM, et al. Theophylline -an immunomodulatory role in asthma? Am Rev Respir Dis 1993; 147: 518–23
Borger P, Postma DS, Vellenga E, et al. Regulation of asthma-related T-cell cytokines by the cyclic AMP-dependent signalling pathway. Clin Exp Immunol 2000; 30(7): 920–6
Borger P, Kauffman HF, Postma DS, et al. Interleukin-4 gene expression in activated human T lymphocytes is regulated by the cyclic adenosine monophosphate-dependent signalling pathway. Blood 1996; 87: 691–8
Borger P, Vellenga E, Gringhuis SI, et al. Prostaglandin E2 differentially modulates interleukin-5 gene expression in activated human T lymphocytes depending on the costimulatory signal. J Allergy Clin Immunol 1998; 101: 231–40
Borger P, Kauffman HF, Vijgen JLJ, et al. Activation of the cAMP-dependent signalling pathway down-regulates the expression of interleukin-3 and granulocyte-macrophage colony-stimulating factor in activated human T lymphocytes. Exp Hematol 1996; 24: 108–15
Romagnami S. The role of lymphocytes in allergic disease. J Allergy Clin Immunol 2000; 105: 399–408
Nicholson CD, Shahid M. Inhibitors of cyclic nucleotide phosphodiesterase isoenzymes: their potential utility in the therapy of asthma. Pulm Pharmacol 1994; 7: 1–17
Spina D. PDE4 inhibitors in the treatment of inflammatory lung disease. Drugs 2003; 63: 2575–94
Beavo J, editor. Molecular pharmacology of cell regulation: cyclic nucleotide phosphodiesterase structure and drug action. New York: Wiley, 1990: 243–66
Soderling SH, Bayuga SJ, Beavo JA. Identification and characterization of a novel family of cyclic nucleotide phosphodiesterases. J Biol Chem 1998; 273: 15553–8
Soderling SH, Bayuga SJ, Beavo JA. Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase. Proc Natl Acad Sci U S A 1998; 95: 8991–6
Stieff CG. Phosphodiesterase inhibitors in the treatment of erectile dysfunction. Drugs Today (Barc) 2000; 36: 93–9
Giembycz MA, Dent G. Prospects for selective cyclic phosphodiesterase inhibitors in the treatment of bronchial asthma. Clin Exp Allergy 1992; 22: 337–44
Conti M, Swinnen JV. Structure and function of the rolipram-sensitive, low Km cyclic AMP phosphodiesterase: a family of highly related proteins. In: Houslay MD, Torphy TJ, editors. Phosphodiesterase isoenzymes: molecular targets for novel antiasthma agents. Am J Respir Crit Care Med 1998; 157: 351–70
Torphy TJ, Barnette MS, Underwood DC, et al. Ariflo (SB207499), a second generation phosphodiesterase 4 inhibitor for the treatment of asthma and COPD: from concept to clinic. Pulm Pharmacol Ther 1999; 12: 131–5
Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol 1986; 39: 177–253
Green RH, Brightling CE, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet 2002; 360: 1715–21
Jatakanon A, Lim S, Barnes PJ. Changes in sputum eosinophils predict loss of asthma control. Am J Respir Crit Care Med 2000; 161: 64–72
Synek M, Beasley R, Frew AJ, et al. Cellular infiltration of the airways in asthma of varying severity. Am J Respir Crit Care Med 1996; 154: 224–30
Alves AC, Pires ALA, Cruz HN, et al. Selective inhibition of phosphodiesterase type IV suppresses the chemotactic responsiveness of rat eosinophils in vitro. Eur J Pharmacol 1996; 312: 852–61
Hatzelmann A, Tenor H, Schudt C. Differential effects of non-selective and selective phosphodiesterase inhibitors on human eosinophil functions. Br J Pharmacol 1995; 114: 821–31
Dent G, Giembycz MA, Evans PM, et al. Suppression of human eosinophil respiratory burst and cyclic AMP hydrolysis by inhibitors of type IV phosphodiesterase: interaction with the beta adrenoreceptor agonist albuterol. J Pharmacol Exp Ther 1994; 271: 1167–74
Ohta K, Yamashita N. Apoptosis of eosinophils and lymphocytes in allergic inflammation. J Clin Immunol 1999; 104: 14–21
Takeuchi M, Tatsumi Y, Kitaichi K, et al. Selective phosphodiesterase inhibitors reduce the prolonged survival of eosinophils stimulated by granulocyte-macrophage colony-stimulating factor. Biol Pharm Bull 2002; 25: 184–7
Souness JE, Aldous D, Sargent C. Immunosuppressive and anti-inflammatory effects of cyclic AMP phosphodiesterase (PDE) type 4 inhibitors. Immunopharmacology 2000; 47: 127–62
Liu J, Munoz NM, Meliton AY, et al. β2-Integrin adhesion caused by eotaxin but not IL-5 is blocked by PDE-4 inhibition and β2-adrenoceptor activation in human eosinophils. Pulm Pharmacol Ther 2004; 17: 73–9
Corrigan CJ, Hartneil A, Kay AB. T lymphocyte activation in acute severe asthma. Lancet 1988; I: 1129–32
Azzawi M, Johnston PW, Majumdar S, et al. T lymphocytes and activated eosinophils in airway mucosa in fatal asthma and cystic fibrosis. Am Rev Respir Dis 1992; 145: 1477–82
Busse WW, Lemanske Jr RF. Asthma. N Engl J Med 2001; 344: 350–62
Lemanske Jr RF, Busse WW. Asthma. JAMA 1997; 278: 1855–73
Szefler SJ, Leung DY. Glucocorticoid resistant asthma: pathogenesis and clinical implications for management. Eur Respir J 1997; 10: 1640–7
Spahn JD, Szefler SJ, Surs W, et al. A novel action of IL-13: induction of diminished monocyte glucocorticoid receptor-binding affinity. J Immunol 1996; 157: 2654–9
Essayan DM, Kagey-Sobotka A, Lichtenstein LM, et al. Differential regulation of human-antigen specific Th1 and Th2 lymphocyte responses by isoenzyme selective cyclic nucleotide phosphodiesterase inhibitors. J Pharmacol Exp Ther 1997; 282: 505–12
Crocker IC, Townley RG, Khan MM. Phosphodiesterase inhibitors suppress proliferation of peripheral blood mononuclear cells and IL-4 and IL-5 secretion by human T-helper type 2 cells. Immunopharmacology 1996; 31: 223–35
Martin RJ, Cicutto LC, Smith HR, et al. Airways inflammation in nocturnal asthma. Am Rev Respir Dis 1991; 143: 351–7
Laitinen LA, Laitinen A, Haahtela T. Airway mucosal inflammation even in patients with newly diagnosed asthma. Am Rev Respir Dis 1993; 147: 697–704
Sur S, Crotty TB, Kephart GM, et al. Sudden onset fatal asthma: a distinct entity with few eosinophils and relatively more neutrophils in the airway submucosal. Am Rev Respir Dis 1993; 148: 713–9
Jatakanon A, Uasuf C, Maziak W, et al. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med 1999; 160: 1001–8
Kamath AV, Pavord ID, Ruparelia PR, et al. Is the neutrophil the key effector cell in severe asthma? Thorax 2005; 60: 529–30
Parsey MV, Tuder R, Abraham E. Neutrophils are major contributors to intraparenchymal lung IL-1 expression after haemorrhage and endotoxaemia. J Immunol 1998; 160: 1007–13
Abraham E. Corticosteroids and the neutrophil: cutting both ways. Crit Care Med 1999; 27: 2583–4
Fahy JV. Remodelling of the airway epithelium in asthma. Am J Respir Crit Care Med 2001; 164: S46–51
Tenor H, Schudt C. Analysis of PDE isoenzyme profiles in cells and tissues by pharmacological methods. In: Schudt C, Dent G, Rabe KF, editors. Phosphodiesterase inhibitors. San Diego (CA): Academic Press, 1996: 21–40
Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 Inhibitor Roflumilast in vitro. J Pharmacol Exp Ther 2001; 297: 267–79
Bloemen PG, van den Tweel MC, Henricks PA, et al. Increased cAMP levels in stimulated neutrophils inhibit their adhesion to human bronchial epithelial cells. Am J Physiol 1997; 272: L580–7
Dent G, Giembycz MA. Selective phosphodiesterase inhibitors in the therapy of asthma. Clin Immunother 1995; 3: 423–37
Torphy TJ, Murray KJ, Arch JRS. Selective phosphodiesterase isoenzyme inhibitors. In: Page CP, Metzger WJ, editors. Drugs and the lung. New York: Raven Press, 1994: 397–447
Eskandari N, Wickramasinghe T, Peachell PT. Effects of phosphodiesterase inhibitors on interleukin-4 and interleukin-13 generation from human basophils. Br J Pharmacol 2004; 142: 1265–72
Banner KH, Moriggi E, Da Ros B, et al. The effect of selective phosphodiesterase 3 and 4 isoenzyme inhibitors and established anti-asthma drugs on inflammatory cell activation. Br J Pharmacol 1996; 119(6): 1255–61
Durham SR, Carrol M, Walsh GM, et al. Leukocyte activation in allergen-induced late-phase asthmatic reactions. N Engl J Med 1984; 311: 1398–402
Gin W, Shaw RJ, Kay AB. Airway reversibility after prednisolone therapy in chronic asthma is associated with alterations in leukocyte function. Am Rev Respir Dis 1985; 132: 1199–203
Gosset P, Tsicopoulos A, Wallaert B, et al. Tumour necrosis factor alpha and interleukin-6 production by human mononuclear phagocytes from allergic asthmatics after IgE-dependent stimulation. Am Rev Respir Dis 1992; 146: 768–74
Broide DH, Gleich GJ, Cuomo AJ, et al. evidence of on going mast cell and eosinophil degranulation in symptomatic asthma airway. J Allergy Clin Immunol 1991; 88: 637–48
Barnette MS, Christensen SB, Underwood DC, et al. Phosphodiesterase 4: biological underpinnings of the design of improved inhibitors. Pharmacol Res Commun 1997; 8: 65–73
Souness JE, Griffen M, Maslen C, et al. Evidence that cAMP phosphodiesterase inhibitors suppress TNF-α generation from human monocytes by interacting with a ‘low affinity’ phosphodiesterase 4 conformer. Br J Pharmacol 1996; 118: 649–58
Ford PA, Giembycz MA, Tetley TD. Cilomilast (Ariflo™) when combined with salbutamol, has an additive effect on suppression of TNF-α release from human lung macrophages. Am J Respir Crit Care Med 2003; 168: A317
Holt PG. Antigen presentation in the lung. Am J Respir Crit Care Med 2000; 162: S151–6
Dunhill MS, Massarella GR, Anderson JA. A comparison of the quantitative anatomy of the bronchi in normal subjects, in status asthmaticus, in chronic bronchitis, and in emphysema. Thorax 1960; 24: 176–9
Cho SH, Seo JY, Coi DC, et al. Pathological changes according to the severity of asthma. Clin Exp Allergy 1996; 26: 1210–9
Bai TR, Mak JCW, Barnes PJ. A comparison of beta-adrenergic receptors and in vitro relaxant responses to isoproterenol in asthmatic airway smooth muscle. Am J Respir Cell Mol Biol 1992; 6: 647–51
Bai TR. Abnormalities in airway smooth muscle in fatal asthma. Am Rev Respir Dis 1990; 141: 552–7
Leung SY, Eynott P, Noble A, et al. Resolution of allergic airways inflammation but persistence of airway smooth muscle proliferation after repeated allergen exposures. Clin Exp Allergy 2004; 34: 213–20
Mehats C, Jin CSL, Wahlstrom J, et al. PDE4D plays a critical role in the control of airway smooth muscle contraction. FASEB J 2003; 17: 1831–41
Le Jeune IR, Shepherd M, Van Heeke G, et al. Cyclic AMP-dependent transcriptional up-regulation of phosphodiesterase 4D5 in human airway smooth muscle cells: identification and characterization of a novel PDE4D5 promoter. J Biol Chem 2002; 277: 35980–9
Tomlinson PR, Wilson JW, Stewart AG. Salbutamol inhibits the proliferation of human airway smooth muscle cells grown in culture: relationship to elevated cAMP levels. Biochem Pharmacol 1995; 49: 1809–19
Maruno K, Absood A, Said SI. VIP inhibits basal and histamine-stimulated proliferation of human airway smooth muscle. Am J Physiol 1995; 268: L1047–51
Florio C, Martin JG, Styhler A, et al. Antiproliferative effects of prostaglandin E2 in cultured guinea pig tracheal smooth muscle cells. Am J Physiol 1994; 266: L131–7
Noveral JP, Grunstein MM. Adrenergic receptor-mediated regulation of cultured rabbit airway smooth muscle cell proliferation. Am J Physiol 1994; 267: L291–9
Johnson-Mills K, Arauz E, Coffey RG, et al. Effect of CI-930[3- (2H)-pyridazinone-4,5-dihydro-6-[4-(1H-imidazolyl)phenyl]-5-methyl monohydrochloride] and rolipram on human coronary artery smooth muscle cell proliferation. Biochem Pharmacol 1998; 56: 1065–73
George SJ, Dwivedi A. MMPs, cadherins, and cell proliferation. Trends Cardiovasc Med 2004; 14(3): 100–5
Billington CK, Joseph SK, Swan C, et al. 1999 Modulation of human airway smooth muscle proliferation by type 3 phosphodiesterase inhibition. Am J Physiol 1999; 276: L412–9
Palmer D, Tsoi K, Maurice DH. Synergistic inhibition of vascular smooth muscle cell migration by phosphodiesterase 3 and phosphodiesterase 4 inhibitors. Circ Res 1998; 82: 852–61
Goncharova EA, Bilington CK, Irani C, et al. Cyclic AMP-mobilising agents and glucocorticoids modulate human smooth muscle cell migration. Am J Respir Cell Mol Biol 2003; 29: 19–27
Howarth PH, Knox AJ, Amrani Y, et al. Synthetic responses in airway smooth muscle. J Allergy Clin Immunol 2004; 114: S32–50
Lazzeri N, Belvisi MG, Patel HJ, et al. Effects of prostaglandin E2 and camp elevating drugs on GM-CSF release by cultured airway smooth muscle cells. Am J Respir Cell Mol Biol 2001; 24: 44–8
Fuhrmann M, Jahn HU, Seybold J, et al. Identification and function of cyclic nucleotide phosphodiesterase isoenzymes in airway epithelial cells. Am J Respir Cell Mol Biol 1999 Feb; 20(2): 292–302
Lange P, Parner J, Vestbo J, et al. A 15-yearfollow-up study of ventilatory function in adults with asthma. N Engl J Med 1998; 339: 1194–200
Ten Hacken NHT, Postma DS, Timens W. Airway remodelling and long-term decline in lung function in asthma. Curr Opin Pulm Med 2003; 9(1): 9–14
Kumar RK, Foster PS. Modelling allergic asthma in mice. Am J Respir Cell Mol Biol 2002; 27: 267–72
Foda HD, George S, Rollo E, et al. Regulation of gelatinases in human airway smooth muscle cells: mechanism of progelatinase A activation. Am J Physiol 1999; 277: L174–82
Van Eerdwegh P, Little RD, Dupuis J, et al. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature 2002; 418: 426–30
Jongepier H, Boezen HM, Dijkstra A, et al. Polymorphisms of the ADAM 33 gene are associated with accelerated lung function decline in asthma. Clin Exp Allergy 2004; 34: 757–60
Johnson PR, Black JL, Carlin S, et al. The production of extracellular matrix proteins by human passively sensitized airway smooth muscle cells in culture: the effect of beclomethasone. Am J Respir Crit Care Med 2000; 162: 2145–51
Shore SA, Moore PE. Effects of cytokines on contractile and dilator responses of airway smooth muscle. Clin Exp Pharmacol Physiol 2002; 29(10): 859–66
Hirst SJ, Martin JG, Bonacci JV, et al. Proliferative aspects of airway smooth muscle. J Allergy Clin Immunol 2004; 114: S2–17
Ertl RF, Valenti V, Spurzem JR, et al. Prostaglandin E inhibits fibroblast recruitment. Am Rev Respir Dis 1992; 145: A19
Guidry C. Fibroblast contraction on collagen gels requires activation of protein kinase C. J Cell Physiol 1993; 155: 358–67
Kohyama T, Liu X, Wen F-Q, et al. PDE4 inhibitors attenuate fibroblast chemotaxis and contraction of native collagen cells. Am J Respir Cell Mol Biol 2002; 26: 694–701
Martin-Chouly CA, Astier A, Jacob C, et al. Modulation of matrix metalloproteinase production from human lung fibroblasts by type 4 phosphodiesterase inhibitors. Life Sci 2004; 74: 823–40
Barnes PJ. Modulation of neurotransmitter release from airways nerves. In: Raeburn D, Giembycz MA, editors. Airways smooth muscle: structure, innervation and neurotransmission. Basel: Birkhauser Verlag, 1994: 9
Sheng H, Ishii K, Murad F. Generation of an endothelium-derived relaxing factorlike substance in bovine tracheal smooth muscle. Am J Physiol 1991; 260: L489–93
Undem BJ, Meeker SN, Chen J, et al. Inhibition of neurally mediated nonadrenergic, noncholinergic contractions of guinea pig bronchus by isozyme- selective phosphodiesterase inhibitors. J Pharmacol Exp Ther 1994; 271: 811–7
Fernandes LB, Ellis JL, Undem BJ, et al. Potentiation of nonadrenergic, noncholinergic relaxation of human isolated bronchus by selective inhibitors of phosphodiesterase isoenzymes. Am J Respir Crit Care Med 1994; 50: 1384–90
Spina D, Harrison S, Page CP. Regulation by phosphodiesterase isoenzymes of non-adrenergic, non-cholinergic contraction in guinea pig isolated main bronchus. Br J Pharmacol 1995; 116: 2334–40
Holbrook M, Gozzard N, James T, et al. Inhibition of bronchospasm and ozone-induced airway hyperresponsiveness in the guinea-pig by CDP840, a novel phosphodiesterase inhibitor. Br J Pharmacol 1996; 118: 1192–200
Underwood DC, Osborn RR, Novak LB, et al. Inhibition of antigen-induced bronchoconstriction and eosinophil infiltration in the guinea pig by the cyclic AMP-specific phosphodiesterase inhibitor, rolipram. J Pharmacol Exp Ther 1993; 266: 306–13
Underwood DC, Bochnowicz S, Osborn RR, et al. Antiasthmatic activity of the second-generation phosphodiesterase 4 (PDE4) inhibitor SB207499 (Ariflo) in the guinea pig. J Pharmacol Exp Ther 1998; 287: 988–95
Bundschuh DS, Eltze M, Barsig J, et al. In vivo efficacy in airway models of roflumilast, a novel orally active PDE4 inhibitor. J Pharmacol Exp Ther 2001; 297: 280–90
Muise ES, Chute IC, Claveau D, et al. Comparison of inhibition of ovalbumin-induced bronchoconstriction in guinea pigs and in vitro inhibition of TNF-α formation with PDE4 inhibitors. Biochem Pharmacol 2002; 63: 1527–35
Wollin L, Barsig J, Marx D, et al. Inhibition by roflumilast of OVA-induced pulmonary leukotriene B4 and cysteinyl-leukotriene production and bronchoconstriction in OVA-sensitised and challenged guinea pigs [abstract]. Am J Respir Crit Care Med 2003; 168: A766
Schimdt DT, Watson N, Dent G, et al. The effect of selective and non-selective phosphodiesterase inhibitors on allergen- and leukotriene C (4)-induced contractions in passively sensitised human airways. Br J Pharmacol 2000; 131(8): 1607–18
Kumar RK, Foster PS. Modelling allergic asthma in mice. Am J Respir Cell Mol Biol 2002; 27: 267–72
Kumar RK, Herbert C, Thomas PS, et al. Inhibition of airway remodelling by roflumilast and dexamethasone in murine chronic asthma. J Pharmacol Exp Ther 2003; 307: 349–55
Compton CH, Cedar E, Nieman RB, et al. Ariflo improves pulmonary function in patients with asthma: results of a study in patients taking corticosteroids. Am J Respir Crit Care Med 1999; 159: A522
Compton CH, Gubb J, Cedar E, et al. The efficacy of Ariflo, a second generation PDE4 inhibitor in patients with asthma. Am J Respir Crit Care Med 1999; 159: A806
Bousquet J, Aubier M, Izquierdo JL, et al. Roflumilast: a new orally active, selective PDE4 inhibitor has equivalent efficacy as inhaled beclomethasone dipropionate in the treatment of asthma. Am J Respir Crit Care Med 2003; 168: D094
Izquierdo JL, Bateman ED, Villasante C, et al. Long term efficacy and safety over one year of once daily Roflumilast, a new, orally active, selective PDE4 inhibitor, in asthma. Am J Respir Crit Care Med 2003; 168: C104
Nieman RB, Fisher BD, Amit O, et al. SB 207499 (Ariflo™), a second generation, selective, oral phosphodiesterase type 4 (PDE4) inhibitor, attenuates exercise induce bronchoconstriction in patients with asthma. Am J Respir Crit Care Med 1998; 157: A413
Timmer W, Leclerc V, Birraux G, et al. The new phosphodiesterase 4 inhibitor roflumilast is efficacious in exercise-induced asthma and leads to suppression of LPS-stimulated TNF-alpha ex vivo. J Clin Pharmacol 2002; 42: 297–303
Coquerot O, Boichot E, Lagente V. Selective type IV phosphodiesterase inhibitors prevent IL-4 induced IgE production by human peripheral blood mononuclear cells. Clin Exp Allergy 1997; 27(7): 816–23
Kleine-Tebbe J, Wicht L, Gagne H, et al. Inhibition of IgE-mediated histamine release from human peripheral leukocytes by selective phosphodiesterase inhibitors. Agents Actions 1992; 36(3–4): 200–6
Bardin PG, Schalkwyk EM, van Heerden K, et al. Roflumilast, a new orally active, selective PDE4 inhibitor shows dose dependant inhibitory effects on allergen-induced late asthmatic reaction. Am J Respir Crit Care Med 2003; 168: C104
Profita M, Chiappara G, Mirabella F, et al. Effect of cilomilast (Ariflo) on TNF-α, IL-8 and GM-CSF release by airway cells of patients with COPD. Thorax 2003; 58: 573–9
Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol 2001; 108 (Pt 2): S147–334
Demoly P, Allaert FA, Lecasble M, et al. PRAGMA. Validation of the classification of ARIA (allergic rhinitis and its impact on asthma). Allergy 2003; 58: 672–5
Schmidt BM, Kusma M, Feuring M, et al. The phosphodiesterase 4 inhibitor roflumilast is effective in the treatment of allergic rhinitis. J Allergy Clin Immunol 2001; 108: 530–6
Schultz A, Boris A, Feuring M, et al. Novel approaches in the treatment of allergic rhinitis. Curr Opin Allergy Clin Immunol 2003; 3: 21–7
Murdoch RD, Cowley H, Kelly J, et al. Cilomilast does not potentiate the cardiovascular effects of inhaled salbutamol. Pulm Pharmacol Ther 2002; 15: 521–7
Compton CH, Diggan M, Cedar E, et al. Safety of Ariflo in a 12 month study of patients with asthma. Am J Respir Crit Care Med 2002; 162: A92
Hauns B, Huennemeyer M, Sieberling M, et al. Investigation of the pharmacokinetics of roflumilast and roflumilast N-oxide after single morning or evening oral administration of 500μg in healthy subjects: an open, randomised, two-period crossover study. Am J Respir Crit Care Med 2003; 116: A92
Obernolte R, Ratzliff J, Baecker PA, et al. Multiple splice variants of phosphodiesterase PDE4C cloned from human lung and testes. Biochem Biophys Acta 1997; 1353: 287–97
Manning CD, Burman M, Christensen SB, et al. Suppression of human inflammatory cell function by subtype selective PDE4 inhibitors correlates with inhibition of PDE4A and PDE4B. Br J Pharmacol 1999; 128: 1393–8
Robichaud A, Tattersall FD, Choudhury I, et al. Emesis induced by inhibitors of type IV cyclic nucleotide phosphodiesterase (PDE IV) in the ferret. Neuropharmacol 1999; 38: 289–97
Robichaud A, Savoie C, Stamatiou PB, et al. Assessing the emetic potential of PDE4 inhibitors on rats. Br J Pharmacol 2002; 135: 113–8
Robichaud A, Stamatiou PB, Jin SLC, et al. Deletion of phosphodiesterase 4D in mice shortens α-2 adrenoreceptor mediated anaesthesia: a behavioural surrogate of emesis. J Clin Invest 2002; 110: 1045–52
Hansen G, Jin S, Umetsu DT, et al. Absence of muscarinic Cholinergic airway responses in mice deficient in the cyclic nucleotide phosphodiesterase PDE4D. Proc Natl Acad Sci U S A 2000; 97: 6245–6
Mehats C, Jin CS-L, Wahlstrom J, et al. PDE4D plays a critical role in the control of airway smooth muscle contraction. FASEB J 2003; 17: 1831–41
Moffat JD, Cocks TM, Page CP. Role of the epithelium and acetylcholine in mediating the contraction to 5-hydroxytryptamine in the mouse isolated trachea. Br J Pharmacol 2004; 141(7): 1159–66
Ariga M, Neitzert B, Nakae S, et al. Nonredundant function of phosphodiesterases 4D and 4B in neutrophil recruitment to the site of inflammation. J Immunol 2004; 173(12): 7531–8
Conti M, Richter W, Mehats C, et al. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem 2003; 278(8): 5493–6
Schneider HH, Schmeichen R, Brezinski M, et al. Stereospecific binding of the antidepressant rolipram to brain protein structures. Eur J Pharmacol 1986; 127: 105–15
Perez-Torres S, Miro X, Palacios JM, et al. Phosphodiesterase type 4 isoenzymes expression in human brain examined by in situ hybridisation histochemistry and [3H]rolipram binding autoradiography: comparison with monkey and rat brain. J Chem Neuroanat 2000; 20: 349–74
Souness JE, Rao S. Proposal for pharmacologically distinct conformers of PDE4 cAMP phosphodiesterases. Cell Signal 1997; 9: 227–36
Souness JE, Griffen M, Maslen C, et al. Evidence that cAMP phosphodiesterase inhibitors suppress TNF-α generation from human monocytes by interacting with a ‘low affinity’ phosphodiesterase 4 conformer. Br J Pharmacol 1996; 118: 649–58
Barnette MS, Christensen SB, Underwood DC, et al. Phosphodiesterase 4: biological underpinnings of the design of improved inhibitors. Pharmacol Rev Commun 1997; 8: 65–73
Kuss H, Hoefgen N, Johanssen S, et al. The in vivo efficacy in airway disease of N-(3,5-dichloropyrid-4-yl)-[1-(4-flurobenzyl)-5-hydroxy-indole-3-yl]-glyoxylic acid amide (AWI 12–281), a selective phosphodiesterase inhibitor for inhaled administration. J Pharmacol Exp Ther 2003; 307: 373–85
Soares AC, Souza DG, Pinho V, et al. Impaired host defence to Klebsiella pneumoniae infection in mice treated with the PDE4 inhibitor rolipram. Br J Pharmacol 2003; 140: 855–62
Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Transcript of Pulmonary Allergy Drug Products Advisory Committee meeting, Friday, September 5, 2003. Holiday Inn Gaithersburg, Gaithersburg, Maryland [online]. Available from URL: http://www.fda.gov/ohrms/dockets/ac/03/transcripts/3976T1.htm [Accessed 2006 May 9]
Acknowledgment
Neither Dr Martin nor Dr Reid have any conflict of interest relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martin, N., Reid, P.T. The Potential Role of Phosphodiesterase Inhibitors in the Management of Asthma. Treat Respir Med 5, 207–217 (2006). https://doi.org/10.2165/00151829-200605030-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00151829-200605030-00006