Abstract
Background and objective: The choice of initial highly active antiretroviral therapy (HAART) should take into account the need to balance efficacy, adverse event risk, resistance concerns for the treatment of HIV and treatment costs. Increased risk of coronary heart disease (CHD) may be of special concern in the selection of HAART therapy, because differences in potential CHD risk have been reported for different regimens. This study aimed to estimate the long-term combined effects of HIV disease and antiretroviral (ARV)-related risk for CHD on quality-adjusted survival and healthcare costs for ARV-naive patients.
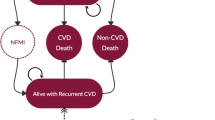
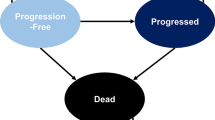
Methods: A previously validated Markov model was updated and supplemented with the Framingham CHD risk equation. In the model, the average patient was male, aged 37 years and had a baseline 10-year CHD risk of 4.6%. Patients started with either lopinavir/ritonavir or unboosted atazanavir as the first protease inhibitor (PI). Clinical trial data were used to estimate the differences between these two therapies. The daily PI costs were $US18.52 for lopinavir/ritonavir and $US22.08 for atazanavir. Other costs were estimated from Medicaid billing databases and average wholesale drug price reports. All model costs were reported as the 2004 present value in US currency. The model’s time horizon reflected a patient’s lifetime, and the perspective of the analysis was that of the healthcare system and did not include indirect costs in the model cost estimates. Various CHD risk levels were tested in the sensitivity analysis.
Results: In the base case, the model predicted a median duration of initial PI regimen of 5.6 years for lopinavir/ritonavir and 3.8 years for atazanavir. Over 10 years, patients who started on atazanavir had 30 additional AIDS events per 100 patients. Only 0.7 additional CHD events per 100 patients occurred for those who started on lopinavir/ritonavir. The model estimated 10-year total healthcare cost savings of $US12 543 per patient in the lopinavir/ritonavir group. The lifetime incremental cost effectiveness of lopinavir/ritonavir versus atazanavir was $US6797 per quality-adjusted life-year gained.
Conclusion: Lopinavir/ritonavir is a highly cost-effective regimen relative to atazanavir for the treatment of HIV. The effect of lopinavir/ritonavir on long-term CHD risk was minimal compared with the increased risk of AIDS/death projected for a less efficacious first PI regimen. The cost of lipid-lowering drugs and treatment of CHD for patients taking the lopinavir/ritonavir regimen was only 1.2% of the cost of AIDS care per person, which was too small to have a significant effect on the overall cost savings with lopinavir/ritonavir therapy. Thus, a decision to forgo potency and durability in an ARV regimen for an ARVnaive patient in favour of a less potent regimen with an improved lipid profile may prove to be costly over time, in terms of both budget impact and life expectancy.




Similar content being viewed by others
References
Guidelines for the use of antiretroviral agents in HIV-1 -infected adults and adolescents. DHHS Panel on Antiretroviral Guidelines, October 10, 2006. Office of AIDS Research Advisory Council [online]. Available from URL: http://AID-Sinfo.nih.gov [Accessed 2006 Nov 7]
FDA Center for Drug Evaluation & Research. Application 21-567 Statistical Review(s) 2002 [online]. Available from URL: http://www.fda.gov/cder/foi/nda/2003/21-567_Reyataz _Statr.pdf [Accessed 2006 Nov 7]
Drummond M, Schulpher M. Common methodological flaws in economic evaluations. Med Care 2005; 43(7 Suppl.): II5–II14
Simpson KN, Luo MP, Chumney EG, et al. Cost effectiveness of using lopinavir/ritonavir vs nelfinavir as the first highly active antiretroviral regimen for HIV infection. HIV Clinical Trials 2004; 5: 294–304
Wong ND, Wilson PW, Kannel WB. Serum cholesterol as a prognostic factor after myocardial infarction: the Framingham Study. Ann Intern Med 1991; 11(5): 687–93
Ghani AC, de Wolf F, Ferguson NM, et al. Surrogate markers for disease progression in treated HIV infection. J Acquir Immune Defic Syndr 2001; 28(3): 226–31
Ghani AC, Henley WE, Donnelly CA, et al. Comparison of the effectiveness of non-nucleoside reverse transcriptase inhibitor-containing and protease inhibitor-containing regimens using observational databases. AIDS 2001; 15(9): 1133–42
Gathe J, Podzamczer D, Johnson M, et al. Once-daily vs twice-daily lopinavir in antiretroviral-naive patients: 48-week results. 11th Conference on Retroviruses and Opportunistic Infections, San Francisco, CA 2004 Feb, Poster #570
Walmsley S, Bernstein B, King M, et al. Lopinavir-ritonavir vs. nelfinavir for the initial treatment of HIV infection. N Engl J Med 2002; 346(26): 2039–46
Simpson KN. Unpublished Medicaid costs data for South Carolina for years 2001 and 2002 inflated by the Medical Care CPI to 2004 values
Price Probe™ 2004 [online]. Available from URL: http://www.firstdatabank.com/reference_products/price_probe [Acessed 2006 Dec 1]
Dolan P. Modeling valuations for EuroQoL health states. Med Care 1998; 35: 1095–108
Castiel D, Herve C, Gaillard M, et al. Cost-utility analysis of early thrombolytic therapy. Pharmacoeconomics 1992; 1(6): 438–42
d’Arminio A, Sabin CA, Phillips AN, et al. Cardio- and cerebrovascular events in HIV-infected persons. AIDS 2004; 18(13): 1811–7
Acknowledgements
This study was funded by a grant from Abbott Laboratories to the Medical University of South Carolina. Drs Luo, King and Brun are employees of Abbott. The other authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simpson, K.N., Luo, M.P., Chumney, E.C. et al. Cost Effectiveness of Lopinavir/Ritonavir Compared with Atazanavir in Antiretroviral-Naive Patients. Clin. Drug Investig. 27, 67–74 (2007). https://doi.org/10.2165/00044011-200727010-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200727010-00006