Abstract
Background and objective: Neuropsychiatric symptoms and behavioural disturbances occur in most patients with Alzheimer’s disease (AD), are a source of stress for caregivers, and are the primary cause of patient institutionalisation. These symptoms often are treated with psychotropic medications. However, adverse drug interactions, adverse effects and nursing home regulations make reducing the use of psychotropic medications in elderly AD patients an important goal of therapy. Fifty-two-week data including data from a 26-week prospective open-label, multicentre study and its 26-week open-label extension were analysed.
Patients and methods: 173 patients with moderate to severe AD residing in nursing homes were treated with rivastigmine 1.5–6mg twice daily. In the study, psychotropic drug use and behavioural symptoms were measured at baseline and at 52 weeks.
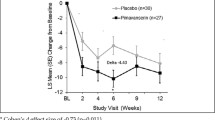
Results: Results showed that 40% of patients who were receiving psychotropic medications at baseline had discontinued use or reduced their dose of psychotropic medications at week 52. Furthermore, significant improvements were observed from baseline in 10 of the 12 behavioural domains of the Nursing Home version of the Neuropsychiatric Inventory, including delusions (mean change from baseline −2.0; p = 0.002), hallucinations (mean change −3.1; p < 0.001), anxiety (mean change −1.1; p = 0.014), and euphoria (mean change −3.2; p = 0.006).
Conclusion: These data suggest favourable tolerability, behavioural and pharmacoeconomic outcomes in nursing home residents with AD who are treated with rivastigmine.





Similar content being viewed by others
References
Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol 2003; 60(8): 1119–22
Mega MS, Cummings JL, Fiorello T, et al. The spectrum of behavioral changes in Alzheimer’s disease. Neurology 1996; 46(1): 130–5
Chen JC, Borson S, Scanlan JM. Stage-specific prevalence of behavioral symptoms in Alzheimer’s disease in a multi-ethnic community sample. Am J Geriatr Psychiatry 2000; 8(2): 123–33
Lyketsos CG, Steinberg M, Tschanz JT, et al. Mental and behavioral disturbances in dementia: findings from the Cache County study on memory in aging. Am J Psychiatry 2000; 157(5): 708–14
Levy ML, Cummings JL, Kahn-Rose R. Neuropsychiatric symptoms and cholinergic therapy for Alzheimer’s disease. Gerontology 1999; 45Suppl. 1: 15–22
Kaufer DI, Cummings JL, Christine D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc 1998; 46(2): 210–5
Gurvich T, Cunningham JA. Appropriate use of psychotropic drugs in nursing homes. Am Fam Physician 2000; 61(5): 1437–46
Risperdal [package insert]. Titusville (NJ): Janssen Pharmaceutica Products LP, 2005
Zyprexa [package insert]. Indianapolis (IN): Eli Lilly and Company, 2005
Farlow MR, Hake A, Messina J, et al. Response of patients with Alzheimer disease to rivastigmine treatment is predicted by the rate of disease progression. Arch Neurol 2001; 58(3): 417–22
Burns A, Spiegel R, Quaig P. Benefits of rivastigmine in patients with severe Alzheimer’s disease (AD). 41st Annual Meeting of the American College of Neuropsychopharmacology; 2002 Dec 8–12; San Juan, Puerto Rico
McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 2000; 356(9247): 2031–6
Giladi N, Shabtai H, Gurevich T, et al. Rivastigmine (Exelon) for dementia in patients with Parkinson’s disease. Acta Neurol Scand 2003; 108(5): 368–73
Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med 2004; 351(24): 2509–18
Moretti R, Torre P, Antonello RM, et al. Rivastigmine in vascular dementia. Expert Opin Pharmacother 2004; 5(6): 1399–410
Grossberg GT, Stahelin HB, Messina JC, et al. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty-two classes of medications. Int J Geriatr Psychiatry 2000; 15(3): 242–7
McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34(7): 939–44
Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44(12): 2308–14
Cummings JL, Anand R, Koumaras B, et al. Rivastigmine provides behavioral benefits to Alzheimer’s disease patients residing in a nursing home: findings from a 26-week trial [abstract no. S79.002]. Neurology 2000; 54: A468
Aupperle PM, Koumaras B, Chen M, et al. Long-term effects of rivastigmine treatment on neuropsychiatric and behavioral disturbances in nursing home residents with moderate to severe Alzheimer’s disease: results of a 52-week open-label study. Curr Med Res Opin 2004; 20(10): 1605–12
Motsinger CD, Perron GA, Lacy TJ. Use of atypical antipsychotic drugs in patients with dementia. Am Fam Physician 2003; 67(11): 2335–40
Cadieux RJ. Drug interactions in the elderly: how multiple drug use increases risk exponentially. Postgrad Med 1989; 86(8): 179–86
Katona CL. Psychotropics and drug interactions in the elderly patient. Int J Geriatr Psychiatry 2001; 16Suppl. 1: S86–90
Polinsky RJ. Clinical pharmacology of rivastigmine: a newgeneration acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin Ther 1998; 20(4): 634–47
Bentue-Ferrer D, Tribut O, Polard E, et al. Clinically significant drug interactions with cholinesterase inhibitors: a guide for neurologists. CNS Drugs 2003; 17(13): 947–63
Desai AK. Use of psychopharmacologic agents in the elderly. Clin Geriatr Med 2003; 19(4): 697–719
Frels C, Williams P, Narayanan S, et al. Iatrogenic causes of falls in hospitalised elderly patients: a case-control study. Postgrad Med J 2002; 78(922): 487–9
FDA public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. Food and Drug Administration. Available from URL: www.fda.gov/cder/drfxg/advisory/antipsychotics.htmDate [Accessed 2005 May 23]
Darvesh S, Grantham DL, Hopkins DA. Distribution of butyrylcholinesterase in the human amygdala and hippocampal formation. J Comp Neurol 1998; 393(3): 374–90
Rosier M, Retz W, Retz-Junginger P, et al. Effects of two-year treatment with the cholinesterase inhibitor rivastigmine on behavioural symptoms in Alzheimer’s disease. Behav Neurol 1998; 11(4): 211–6
Corey-Bloom J, Anand R, Veach J. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int J Geriatr Psychopharmacol 1998; 1: 55–65
Tariot PN, Solomon PR, Morris JC, et al. A 5-month, randomized, placebo-controlled trial of galantamine in AD. Neurology 2000; 54(12): 2269–76
Cummings JL, Schneider L, Tariot PN, et al. Reduction of behavioral disturbances and caregiver distress by galantamine in patients with Alzheimer’s disease. Am J Psychiatry 2004; 161(3): 532–8
Tariot PN, Cummings JL, Katz IR, et al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of donepezil in patients with Alzheimer’s disease in the nursing home setting. J Am Geriatr Soc 2001; 49(12): 1590–9
Rogers SL, Doody RS, Mohs RC, et al. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Arch Intern Med 1998; 158(9): 1021–31
Acknowledgements
This research was sponsored by Novartis Pharmaceuticals Corp.
Keith Edwards has received research grant funds from Novartis for this study, he is on the Novartis Speakers’ bureau and he has been a Consultant to Novartis on various other studies for which he has received payment. He owns no stock or bonds nor does anyone in his family work for Novartis nor do they own any stock or bonds. The co-authors (Barbara Koumaras, Michael Chen, Ibrahim Gunay and Dario Mirski) are all employees of Novartis Pharmaceuticals Corporation.
* Rivastigmine Nursing Home Study Team: Ellen Binder, MD, Washington University School of Medicine, St Louis, MO; Jeffrey L. Cummings, MD, UCLA Department of Neurology, Los Angeles, CA; Marshal Folstein, MD, Department of Psychiatry, New England Medical Center, Boston, MA; Elsa M. Zayas, MD, St Louis University Medical Center, St Louis, MO; Raymond Ownby, MD, University of Miami School of Medicine, Miami, FL; Charles H. Merideth, MD, Affiliated Research Institute, San Diego, CA; Douglas W. Scharre, MD, Ohio State University Department of Neurology, Columbus, OH; Pierre Tariot, MD, Monroe Community Hospital, Rochester, NY; Keith Edwards, MD, Alzheimers Diagnostic Center, Bennington, VT; Peter M. Aupperle, MD, Comprehensive Services on Aging UMDNJ, Piscataway, NJ; Jay. M. Ellis, DO, Berkshire Neurological Associates, Pittsfield, MA; Jorg J. Pahl, MD, Pahl Brain Associates, Oklahoma City, OK; Ari Kiev, MD, Social Psychiatry Research Institute, Englewood, NJ and New York, NY.
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
Edwards, K., Koumaras, B., Chen, M. et al. Long-Term Effects of Rivastigmine Treatment on the Need for Psychotropic Medications in Nursing Home Patients with Alzheimer’s Disease. Clin. Drug Investig. 25, 507–515 (2005). https://doi.org/10.2165/00044011-200525080-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200525080-00003