Abstract
Objective: To assess the potential effects of food on the pharmacokinetics and tolerability/safety of ximelagatran, an oral direct thrombin inhibitor developed for the prevention and treatment of thromboembolic disease that is rapidly bioconverted to its active form, melagatran.
Design and study participants: In two open-label, randomised, crossover studies, healthy male and female volunteers received oral ximelagatran as a single 24mg tablet (study 1, n = 30) or a single 36mg tablet (study 2, n = 50). Potential effects of food on the pharmacodynamics (activated partial thromboplastin time; APTT) of the 36mg tablet were also investigated in study 2.
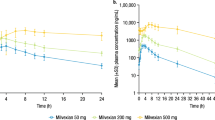
Results: For the 24mg tablet, the 90% confidence intervals (CIs) and least-squares mean estimates for the ratio of the tablet with food to the tablet without food fell within the predefined bounds demonstrating no effect on area under the melagatran concentration-time curve (AUC ratio = 0.94 [90% CI 0.90, 0.99]) or maximum plasma concentration (Cmax ratio = 0.88 [90% CI 0.82, 0.95]). The same result was observed for the 36mg tablet (AUC ratio = 1.07 [90% CI 1.03, 1.12]; Cmax ratio = 1.05 [90% CI 0.98, 1.12]). Melagatran AUC normalised for differences in bodyweight was comparable between women and men administered the 24mg or 36mg tablet without food. In addition, food did not clinically significantly alter the melagatran-induced prolongation of the APTT of the 36mg tablet. Ximelagatran was well tolerated with or without food.
Conclusion: The pharmacokinetics (AUC, Cmax), pharmacodynamics (APTT) and tolerability of melagatran after administration of oral ximelagatran tablets were not affected by food.






Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Hrebickova L, Nawarskas JJ, Anderson JR. Ximelagatran: a new oral anticoagulant. Heart Dis 2003; 5: 397–408
Da Silva MS, Sobel M. Anticoagulants: to bleed or not to bleed, that is the question. Semin Vasc Surg 2002; 15: 256–67
Pinede L, Duhaut P, Ninet J. Management of oral anticoagulants in the treatment of venous thromboembolism. Eur J Intern Med 2001; 12: 75–85
Eriksson H, Wåhlander K, Gustafsson D, et al. A randomized, controlled, dose-guiding study of the oral direct thrombin inhibitor ximelagatran compared with standard therapy for the treatment of acute deep vein thrombosis: THRIVE I. J Thromb Haemost 2003; 1: 41–7
Eriksson BI, Bergqvist D, Kalebo P, et al. Ximelagatran and melagatran compared with dalteparin for prevention of venous thromboembolism after total hip or knee replacement: the METHRO II randomised trial. Lancet 2002; 360: 1441–7
Eriksson BI, Agnelli G, Cohen AT, et al. on behalf of the EXPRESS Study Group. The direct thrombin inhibitor melagatran followed by oral ximelagatran compared with enoxaparin for the prevention of venous thromboembolism after total hip or knee replacement: the EXPRESS study. J Thromb Haemost 2003; 1: 2490–6
Francis CW, Berkowitz SD, Comp PC, et al. for the EXULT A Study Group. Comparison of ximelagatran with warfarin for the prevention of venous thromboembolism after total knee replacement. N Engl J Med 2003; 349: 1703–12
Executive Steering Committee on behalf of the SPORTIF III Investigators. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet 2003; 362: 1691–8
Schulman S, Wåhlander K, Lundström T, et al. for the THRIVE III Investigators. Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. N Engl J Med 2003; 349: 1713–21
Wallentin L, Wilcox R, Weaver WD, et al. Oral ximelagatran for secondary prophylaxis after myocardial infarction: the randomized controlled ESTEEM trial. Lancet 2003; 362: 789–97
Gustafsson D, Elg M. The pharmacodynamics and pharmacokinetics of the oral direct thrombin inhibitor ximelagatran and its active metabolite melagatran: a mini-review. Thromb Res 2003; 109: S9–15
Sarich TC, Johansson S, Schützer K-M, et al. The pharmacokinetics and pharmacodynamics of ximelagatran, an oral direct thrombin inhibitor, are unaffected by a single dose of alcohol. J Clin Pharmacol 2004; 44: 388–93
Sarich TC, Schützer KM, Dorani H, et al. No pharmacokinetic or pharmacodynamic interaction between atorvastatin and the oral direct thrombin inhibitor ximelagatran. J Clin Pharmacol 2004; 44: 928–34
Teng R, Sarich TC, Eriksson JG, et al. A pharmacokinetic study of the combined administration of amiodarone and ximelagatran, an oral direct thrombin inhibitor. J Clin Pharmacol 2004; 44: 1063–71
Sarich TC, Schützer KM, Wollbratt M, et al. No pharmacokinetic or pharmacodynamic interaction between digoxin and the oral direct thrombin inhibitor ximelagatran in healthy volunteers. J Clin Pharmacol 2004; 44: 935–41
Dorani H, Schützer K-M, Wollbratt M, et al. No clinically significant interaction between the oral direct thrombin inhibitor ximelagatran and amiodarone, atorvastatin or digoxin [abstract no. P1-98]. Clin Pharmacol Ther 2004; 75(2): 78
Dorani H, Schützer K, Sarich TC, et al. Effect of erythromycin on the pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor ximelagatran and its active form melagatran [abstract]. Clin Pharmacol Ther 2004; 75: P78
Dorani H, Schützer K, Sarich TC, et al. No effect of amoxicillin, doxycycline, and ciprofloxacin on the pharmacokinetics and pharmacodynamics of ximelagatran [abstract no. PI-147]. Clin Pharmacol Ther 2005; 77(2): 46
Dorani H, Schützer K, Sarich TC, et al. The pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor ximelagatran when administered with azithromycin and cefuroxime [abstract no. PII-126]. Clin Pharmacol Ther 2005; 77(2): 83
Johansson LC, Frison L, Logren U, et al. Influence of age on the pharmacokinetics and pharmacodynamics of ximelagatran, an oral direct thrombin inhibitor. Clin Pharmacokinet 2003; 42: 381–92
Eriksson UG, Bredberg U, Gislén K, et al. Pharmacokinetics and pharmacodynamics of ximelagatran, a novel oral direct thrombin inhibitor, in young healthy male subjects. Eur J Clin Pharmacol 2003; 59: 35–43
Larsson M, Logren U, Ahnoff M, et al. Determination of melagatran, a novel direct thrombin inhibitor, in human plasma and urine by liquid chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl 2002; 766: 47–55
Wernevik LC, Nyström P, Johnsson G, et al. Pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor ximelagatran in young healthy Japanese men. Clin Pharmacokinet. In press
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41
Gustafsson D, Nystrom J, Carlsson S, et al. The direct thrombin inhibitor melagatran and its oral prodrug H 376/95: intestinal absorption properties, biochemical and pharmacodynamic effects. Thromb Res 2001; 101: 171–81
Grind M, Hamren B, Baathe S, et al. Pharmacokinetics of the oral direct thrombin inhibitor ximelagatran in patients with nonvalvular atrial fibrillation receiving long-term treatment: a population analysis by nonlinear mixed effect modeling [abstract no. MPI-99]. Clin Pharmacol Ther 2002; 71: P31
Sarich TC, Teng R, Peters GR, et al. No influence of obesity on the pharmacokinetics and pharmacodynamics of melagatran, the active form of the oral direct thrombin inhibitor, ximelagatran. Clin Pharmacokinet 2003; 42: 485–92
Johansson LC, Andersson M, Fager G, et al. No influence of ethnic origin on the pharmacokinetics and pharmacodynamics of melagatran following oral administration of ximelagatran, a novel oral direct thrombin inhibitor, to healthy male volunteers. Clin Pharmacokinet 2003; 42: 475–84
Warfarin Prescribing Information. Pomona (NY): Barr Laboratories Inc., 2002 Nov
Hirsh J. Current anticoagulant therapy: unmet clinical needs. Thromb Res 2003; 109Suppl. 1: S1–8
Acknowledgements
This study was funded by AstraZeneca. The authors acknowledge the assistance of Jane Saiers, PhD, in writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ersdal, E., Schützer, KM., Lönnerstedt, C. et al. No Influence of Food on the Pharmacokinetics, Pharmacodynamics or Tolerability of the 24mg and 36mg Oral Tablet Formulations of Ximelagatran. Clin. Drug Investig. 25, 425–433 (2005). https://doi.org/10.2165/00044011-200525070-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200525070-00001