Abstract
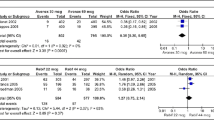
Three interferon-beta (IFNβ) products (Betaferon®, Avonex® and Rebif®) have been shown to be effective in the treatment of relapsing forms of multiple sclerosis, but the optimal dose of each product has not been established. The lack of head-to-head trials comparing the different IFNβs directly has led to comparisons across clinical trials, despite known limitations of such an approach (e.g. differences between studies with regard to patients studied, design, duration and end-point definitions). Furthermore, IFNβ preparations are manufactured and formulated differently and are delivered by different routes of administration; they are thus different with respect to their bioavailability, immunogenicity and specific activities. Nevertheless, the results of a manual review of all major published dose-comparison studies on IFNβ preparations suggest that there may be a dose-response effect below a certain threshold dose for each product. However, once this threshold dose is attained, a ceiling effect exists; higher doses do not provide added clinical benefit, but may be associated with a higher incidence of adverse events. Hence, physicians should weigh the lack of additional clinical benefits of higher IFNβ doses with the increased risk of adverse events when deciding on the appropriate dose of IFNβ for their patients.



Similar content being viewed by others
References
Milo R, Panitch H. Interferons in multiple sclerosis. Harefuah 1994; 126(8): 451–8
Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-la for disease progression in relapsing multiple sclerosis. Ann Neurol 1996; 39: 285–94
PRISMS (Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) Study Group. Randomized double-blind placebo-controlled study of inter-feron β-1a in relapsing/remitting multiple sclerosis. Lancet 1998; 352: 1498–504
Rudick RA, Goodkin DE, Jacobs LD, et al. Impact of interferon beta-1a on neurologic disability in relapsing multiple sclerosis. Neurology 1997; 49: 358–63
The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993; 43: 655–61
The Once Weekly Interferon for MS Study Group (OWIMS). Evidence of interferon β-1a dose response in relapsing-remitting MS. Neurology 1999; 53: 679–86
Paty DW, Li DKB, the UCB MS/MRI Study Group, the IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993; 43: 662–7
Rudick RA, Fischer E, Lee J-C, et al. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing remitting MS. Neurology 1999; 53: 1698–704
The IFNB Multiple Sclerosis Study Group. Interferon beta-1b in the treatment of multiple sclerosis. Final outcome of the randomized controlled trial. Neurology 1995; 45: 1277–85
European Study Group on Interferon β-1b in Secondary Progressive MS. Placebo-controlled multicentre randomised trial of interferon β-1b in the treatment of secondary progressive multiple sclerosis. Lancet 1998; 352: 1491–7
Miller DH, Molyneux PD, Barker GJ, et al. Effect of interferon—beta 1b on magnetic resonance imaging outcomes in secondary progressive multiple sclerosis: results of a European multicenter randomized double-blind placebo-controlled trial. European Study Group on Interferon-beta 1b in secondary progressive multiple sclerosis. Ann Neurol 1999; 46(6): 850–9
Molyneux PD, Kappos L, Polman C, et al. The effect of interferon betalb treatment on MRI measures of cerebral atrophy in secondary progressive multiple sclerosis. Brain 2000; 123: 2256–63
Goodkin DE. The North American study of interferon beta-1b in secondary progressive multiple sclerosis. Abstract. Paper presented at the 52nd Annual Meeting of the American Academy of Neurology, 1 May 2000; San Diego, California, USA
Chofflon M. Recombinant human interferon beta in relapsing-remitting multiple sclerosis: a review of the major clinical trials. Eur J Neurol 2000; 7: 369–80
Jacobs LD, Beck SW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N Engl J Med 2000 343(13): 898–904
European Study Group on Interferon-Beta-1a in MS. Double-blind randomized multicenter dose-comparison study of interferon-beta-1a (Avonex): rationale, design and baseline data. Multiple Sclerosis. In press
European Study Group on Interferon-Beta-1a in MS. A randomised, double-blind, multicentric dose comparison study of interferon-beta-1a (Avonex) for the treatment of relapsing multiple sclerosis. Lancet. In press
Freedman MS. PRISMS 4-year study results. Abstract. Paper presented at the 52nd Annual Meeting of the American Academy of Neurology, 1 May 2000; San Diego, California, USA
Comi G, Filippi M, Barkhof F, et al. Interferon Beta 1a (Rebif) in patients with acute neurological syndromes suggestive of multiple sclerosis: a multi-center, randomized, double-blind, placebo-controlled study. Abstract. Paper presented at the 52nd Annual Meeting of the American Academy of Neurology, 2 May 2000; San Diego, California, USA
Blumhardt LD. Interferon beta-1a (Rebif®) in the treatment of secondary progressive multiple sclerosis. I. Clinical results [abtract no. 37]. Abstract. Presented at the 9th European Neurological Society Meeting, 7 June 1999, Milan Italy
Miller DH, Albert PS, Barkhof F, et al. Guidelines for use of magnetic resonance techniques in monitoring the treatment of multiple sclerosis. Ann Neurol 1996; 39: 6–16
O’Riordan JL, Thompson AT, Kingsley DPE, et al. The prognostic value of brain MRI in clinically isolated syndromes of the CNS. A 10-year follow-up. Brain 1998; 121: 495–503
Cutter GR, Baier Ml, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome. Brain 1999; 122: 871–82
Kalkers NF, Bergers L, de Groot V, et al. Concurrent validity of the MS Functional Composite using MRI as a biological marker. Neurology 2001; 56: 215–9
Knobler RL, Greenstein JI, Johnson KP, et al. Systemic recombinant human interferon beta treatment of relapsing-remitting multiple sclerosis: pilot study analysis and six-year follow-up. J Interferon Res 1993; 13(5): 333–40
Rice GPA, Ebers GC, Lublin FD, et al. Ibuprofen treatment versus gradual introduction of interferon β 1b in patients with multiple sclerosis. Neurology 1999; 52(9): 1893–5
Runkel L, Meier W, Pepinsky RB, et al. Structural and functional differences between glycosylated and non-glycosylated forms of human interferon-β (IFN-β). Pharm Res 1998; 15: 641–9
Rudick RA, Simonian NA, Alam JA, et al. Incidence and significance of neutralizing antibodies to interferon beta-1a in multiple sclerosis. Neurology 1998; 50: 1266–72
Bhatia PK, Mukhopadhyay A. Protein glycosylation: implications for in vivo functions and therapeutic applications. Adv Biochem Eng Biotechnol 1999; 64: 155–201
Jones WE, Quigley K, Parish TH, et al. Evaluation of interferon-beta immunogenicity in mice [abstract P01.075]. Neurology 1998; 50 Suppl. 4: A34
Rudd PM, Woods RJ, Wormald MR, et al. The effects of variable glycosylation on the functional activities of ribonuclease, plasminogen and tissue plasminogen activator. Biochim Biophys Acta 1995; 1248: 1–10
Van den Steen P, Rudd PM, Dwek RA, et al. Concepts and principles of O-linked glycosylation. Crit Rev Biochem Mol Biol 1998; 33: 151–208
Kivisakk P, Alm GV, Fredrikson S, et al. Neutralizing and binding anti-interferon-β (IFN-β) antibodies. A comparison between IFN-β-1a and IFN-β-1b treatment in multiple sclerosis. Eur J Neurol 2000; 7: 27–34
Ross C, Clemmesen KM, Svenson M, et al. Immunogenicity of interferon-β in multiple sclerosis patients: influence of preparation, dosage, dose frequency and route of administration. Ann Neurol 2000; 48: 706–12
Acknowledgements
This study was funded by an educational grant from Biogen, Inc., Cambridge, MA, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clanet, M. Interferon-Beta in the Treatment of Multiple Sclerosis. Clin. Drug Investig. 21, 307–318 (2001). https://doi.org/10.2165/00044011-200121040-00009
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200121040-00009