Abstract
Objective
To evaluate the efficacy and tolerability of desloratadine 5mg once daily, a new, selective, H1-receptor antagonist, for the treatment of patients with seasonal allergic rhinitis (SAR) during the two major pollen seasons in the USA.
Design
Two multicentre, randomised, double-blind, placebo-controlled, parallel-group investigations in patients with SAR are reported, one conducted during the spring (172 and 174 patients in the desloratadine and placebo groups, respectively) and the other during the fall (164 patients each in the desloratadine and placebo groups) allergy season.
Study Participants
Patients 12 years of age or older with clinically symptomatic SAR and a minimum 2-year history of SAR.
Interventions
Desloratadine 5mg or placebo once daily for 14 days following a 1-week screening period.
Main Outcome Measures
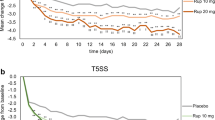
The primary efficacy assessment was the mean change from baseline in the average reflective am/pm total symptom score (TSS) averaged over the 2-week study period.
Results
In both seasons, desloratadine 5mg once daily resulted in a significant improvement in TSS for patients with SAR (p < 0.01 and p = 0.02, respectively) over the 2-week study. Adverse events reported were mild to moderate in severity and similar to placebo. Assessment of sedation and ECG data revealed no clinically significant changes from baseline with desloratadine- or placebo-treated patients.
Conclusion
Desloratadine 5mg once daily was effective and well tolerated in the treatment of symptoms associated with SAR following the first dose of therapy and continuing for the 2-week duration of the study during both the spring and fall allergy seasons.








Similar content being viewed by others
References
American Academy of Allergy Asthma & Immunology. The Allergy Report, Volume 1: Overview of Allergic Diseases. 2000. Milwaukee, WI, American Academy of Allergy, Asthma & Immunology
Schoenwetter WF. Allergic rhinitis: Epidemiology and natural history. Allergy Asthma Proc 2000; 21: 1–6
Nightingale CH. Treating allergic rhinitis with second-generation antihistamines. Pharmacotherapy 1996; 16: 905–14
Corry DB, Kheradmand F. Induction and regulation of the IgE response. Nature 1999; 402: B18–B23
Kreutner W, Hey JA, Anthes J, et al. Preclinical pharmacology of desloratadine, a selective and nonsedating histamine H1 receptor antagonist: 1st communication: Receptor selectivity, antihistaminic activity, and antiallergenic effects. Arzneimittelforschung 2000; 50: 345–52
Affrime M, Gupta S, Banfield C, et al. A pharmacokinetic profile of desloratadine in healthy adults including elderly subjects. Clin Pharmacokinet. In press
Gupta S, Padhi D, Banfield C, et al. The oral bioavailability of desloratadine is unaffected by food, [abstract] Allergy 2000; 55 Suppl. 63: 268
Banfield C, Hunt T, Reyderman L, et al. Lack of interaction between desloratadine and erythromycin. Clin Pharmacokinet. In press
Banfield C, Herron J, Keung A, et al. Desloratadine has no electrocardiographic or pharmacodynamic interactions with ketoconazole. Clin Pharmacokinet. In press
Marino M, Glue P, Herron JM, et al. Lack of electrocardiographic effects of multiple high doses of desloratadine. [abstract] Allergy 2000; 55 Suppl. 63: 279
Hadley JA. Evaluation and management of allergic rhinitis. Med Clin North Am 1999; 83: 13–25
Day JH, Briscoe M, Widlitz MD. Cetirizine, loratadine, or placebo in subjects with seasonal allergic rhinitis: Effects after controlled ragweed pollen challenge in an environmental exposure unit. J Allergy Clin Immunol 1998; 101: 638–45
Gutkowski A, Bedard PM, Del Carpio J, et al. Comparison of the efficacy and safety of loratadine, terfenadine, and placebo in the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol 1988; 81: 902–7
Lockey RF, Widlitz MD, Mitchell DQ, et al. Comparative study of cetirizine and terfenadine versus placebo in the symptomatic management of seasonal allergic rhinitis. Ann Allergy Asthma Immunol 1996; 76: 448–54
Bronsky E, Falliers CJ, Kaiser HB, et al. Effectiveness and safety of fexofenadine, a new nonsedating H1 -receptor antagonist, in the treatment of fall allergies. Allergy Asthma Proc 1998; 19: 135–41
Casale TB, Andrade C, Qu R. Safety and efficacy of once-daily fexofenadine HCl in the treatment of autumn seasonal allergic rhinitis. Allergy Asthma Proc 1999; 20: 193–8
Acknowledgements
Funding was provided by Schering-Plough Research Institute, Kenilworth, New Jersey, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meltzer, E.O., Prenner, B.M. & Nayak, A. Efficacy and Tolerability of Once-Daily 5mg Desloratadine, an H1-Receptor Antagonist, in Patients with Seasonal Allergic Rhinitis. Clin. Drug Investig. 21, 25–32 (2001). https://doi.org/10.2165/00044011-200121010-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200121010-00004