Abstract
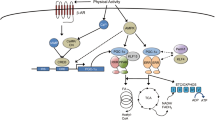
Behavioural and hereditary conditions are known to decrease mitochondrial volume and function within skeletal muscle. This reduces endurance performance, and is manifest both at high- and low-intensity levels of exertion. A programme of regular endurance exercise, undertaken over a number of weeks, produces significant adaptations within skeletal muscle such that noticeable improvements in oxidative capacity are evident, and the related decline in endurance performance can be attenuated. Notwithstanding the important implications that this has for the highly trained endurance athlete, an improvement in mitochondrial volume and function through regular physical activity also endows the previously sedentary and/or aging population with an improved quality of life, and a greater functional independence. An understanding of the molecular and cellular mechanisms that govern the increases in mitochondrial volume with repeated bouts of exercise can provide insights into possible therapeutic interventions to care for those with mitochondrially-based diseases, and those unable to withstand regular physical activity. This review focuses on the recent developments in the molecular aspects of mitochondrial biogenesis in chronically exercising muscle. Specifically, we discuss the initial signalling events triggered by muscle contraction, the activation of transcription factors involved in both nuclear and mitochondrial DNA transcription, as well as the post-translational import mechanisms required for mitochondrial biogenesis. We consider the importance and relevance of chronic physical activity in the induction of mitochondrial biogenesis, with particular emphasis on how an endurance training programme could positively affect the age-related decline in mitochondrial content and delay the progression of age- and physical inactivity-related diseases.



Similar content being viewed by others
References
Pette D, editor. Plasticity of skeletal muscle. Berlin: Walter de Gruyter, 1980
Howald H, Hoppeler H, Claassen H, et al. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflugers Arch 1985; 403: 369–76
Reichmann H, Pette D. A comparative microphotometric study of succinate dehydrogenase activity levels in type I, IIA, and IIB fibres of mammalian and human muscles. Histochemistry 1982; 74: 27–41
Holloszy JO. Biochemical adaptations in muscle: effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 1967; 242: 2278–82
Baldwin KM, Klinkerfuss GH, Terjung RL, et al. Respiratory capacity of white, red, and intermediate muscle: adaptive response to exercise. Am J Physiol 1972; 222: 373–8
Takahashi M, Hood DA. Chronic stimulation-induced changes in mitochondria and performance in rat skeletal muscle. J Appl Physiol 1993; 74: 934–41
Wicks KL, Hood DA. Mitochondrial adaptations in denervated muscle: relationship to muscle performance. Am J Physiol 1991; 260: C841–50
Hoppeler H. Exercise-induced ultrastructural changes in skeletal muscle. Int J Sports Med 1986; 7: 187–204
Durante PE, Mustard KJ, Park S-H, et al. Effects of endurance training on activity and expression of AMP-activated protein kinase isoforms in rat muscles. Am J Physiol 2002; 283: E178–86
Wallace DC. Mitochondrial diseases in man and mouse. Science 1999; 283: 1482–8
Li B, Holloszy JO, Semenkovich CF. Respiratory uncoupling induces delta-aminolevulinate synthase expression through a nuclear respiratory factor-1-dependent mechanism in HeLa cells. J Biol Chem 1999; 274: 17534–40
Biswas G, Adebanjo OA, Freedman BD, et al. Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of interorganelle crosstalk. EMBO J 1999; 18: 522–33
Ohira Y, Cartier LJ, Chen M, et al. Induction of an increase in mitochondrial matrix enzymes in muscle or iron-deficient rats. Am J Physiol 1987; 253: C639–44
Van Deurson J, Heerschap A, Oerlemans F, et al. Skeletal muscles of mice deficient in muscle creatine kinase lack burst activity. Cell 1993; 74: 621–31
Shoubridge EA, Challiss RA, Hayes DJ, et al. Biochemical adaptation in the skeletal muscle of rats depleted of creatine with the substrate analogue beta-guanidinopropionic acid. Biochem J 1985; 232: 125–31
Bergeron R, Ren JM, Cadman KS, et al. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol 2001; 281: E1340–6
Winder WW, Holmes BF, Rubink DS, et al. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol 2000; 88: 2219–26
Hood DA. Invited review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol 2001; 90: 1137–57
Lawrence Jr JC, Salsgiver WJ. Levels of enzymes of energy metabolism are controlled by activity of cultured rat myotubes. Am J Physiol 1983; 244: C348–55
Freyssenet D, Di Carlo M, Hood DA. Calcium-dependent regulation of cytochrome C gene expression in skeletal muscle cells. J Biol Chem 1999; 274: 9305–11
Ojuka EO, Jones TE, Han H-H, et al. Intermittent increases in cytosolic Ca2+ stimulate mitochondrial biogenesis in muscle cells. Am J Physiol 2002; 283: E1040–5
Wu H, Kanatous SB, Thurmond FA, et al. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 2002; 296: 349–52
Scarpulla RC. Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene 2002; 286: 81–9
Nelson BD, Luciakova K, Li R, et al. The role of thyroid hormone and promoter diversity in the regulation of nuclear encoded mitochondrial proteins. Biochim Biophys Acta 1995; 1271: 85–91
Xia Y, Buja LM, McMillin JB. Activation of the cytochrome C gene by electrical stimulation in neonatal rat cardiac myocytes: role of NRF-1 and c-Jun. J Biol Chem 1998; 273: 12593–8
Murakami T, Shimomura Y, Yoshimura A, et al. Induction of nuclear respiratory factor-1 expression by an acute bout of exercise in rat muscle. Biochim Biophys Acta 1998; 1381: 113–22
Puigserver P, Wu Z, Park CW, et al. A cold-inducible coac-tivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998; 92: 829–39
Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 2002; 418: 797–801
Terada S, Goto M, Kato M, et al. Effects of low-intensity exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun 2002; 296: 350–4
Irrcher I, Adhihetty PJ, Sheehan T, et al. PPARγ coac-tivator-1 alpha expression during thyroid hormone and contractile activity-induced mitochondrial adaptations. Am J Physiol 2003; 284(6): C1669–77
Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999; 98: 115–24
Lehman JJ, Barger PM, Kovacs A, et al. Peroxisome prolifer-ator-activated receptor γ coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 2000; 106: 847–56
Mihara K, Omura T. Cytosolic factors in mitochondrial import. Experientia 1996; 52: 1063–8
Chewawiwat N, Yano M, Terada K, et al. Characterization of the novel mitochondrial protein import component, Tom34, in mammalian cells. J Biochem 1999; 125: 721–7
Schneider HC, Berthold J, Bauer MF, et al. Mitochondrial Hsp70/MIM44 complex facilitates protein import. Nature 1994; 371: 768–74
Cogswell AM, Stevens RJ, Hood DA. Properties of skeletal muscle mitochondria isolated from subsarcolemmal and in-termyofibrillar regions. Am J Physiol 1993; 264: C383–9
Takahashi M, Hood DA. Protein import into subsarcolemmal and intermyofibrillar skeletal muscle mitochondria: differential import regulation in distinct subcellular regions. J Biol Chem 1996; 271: 27285–91
Ornatsky OI, Connor MK, Hood DA. Expression of stress proteins and mitochondrial chaperonins in chronically stimulated skeletal muscle. Biochem J 1995; 311: 119–23
Takahashi M, Chesley A, Freyssenet D, et al. Contractile activity-induced adaptations in the mitochondrial protein import system. Am J Physiol 1998; 274: C1380–7
Craig EE, Chesley A, Hood DA. Thyroid hormone modifies mitochondrial phenotype by increasing protein import without altering degradation. Am J Physiol 1998; 275: C1508–15
Koehler CM, Leuenberger D, Merchant S, et al. Human deafness dystonia syndrome is a mitochondrial disease. Proc Natl Acad Sci U S A 1999; 96: 2141–6
Tranebjaerg L, Hamel BC, Gabreels FJ, et al. A de novo missense mutation in a critical domain of the X-linked DDP gene causes the typical deafness-dystonia-optic atrophy syndrome. Eur J Hum Genet 2000; 8: 464–7
Moraes CT. What regulates mitochondrial DNA copy number in animal cells. Trends Genet 2001; 4: 199–205
McCulloch V, Seidel-Rogol BL, Shadel GS. A human mitochondrial transcription factor is related to RNA adenine methyltransferases and binds S-adenosylmethionine. Mol Cell Biol 2002;22: 1116–25
Williams RS. Mitochondrial gene expression in mammalian striated muscle: evidence that variation in gene dosage is the major regulatory element. J Biol Chem 1986; 261: 12390–4
Hood DA, Zak R, Pette D. Chronic stimulation of rat skeletal muscle induces coordinate increases in mitochondrial and nuclear mRNAs of cytochrome-c-oxidase subunits. Eur J Biochem 1989; 179: 275–80
Gordon JW, Rungi AA, Inagaki H, et al. Effects of contractile activity on mitochondrial transcription factor A expression in skeletal muscle. J Appl Physiol 2001; 90: 389–96
Bengtsson J, Gustafsson T, Widegren U, et al. Mitochondrial transcription factor A and respiratory complex IV increase in response to exercise training in humans. Pflugers Arch 2001; 443: 61–6
Ordway GA, Li K, Hand GA, et al. RNA subunit of mitochondrial RNA-processing enzyme is induced by contractile activity in striated muscle. Am J Physiol 1993; 265: C1511–6
Schultz RA, Swoap SJ, McDaniel LD, et al. Differential expression of mitochondrial DNA replication factors in mammalian tissues. J Biol Chem 1998; 273: 3447–51
Puntschart A, Claassen H, Jostarndt K, et al. mRNAs of enzymes involved in energy metabolism and mtDNA are increased in endurance-trained athletes. Am J Physiol 1995; 269: C619–25
Connor MK, Bezborodova O, Escobar CP, et al. Effect of contractile activity on protein turnover in skeletal muscle mitochondrial subfractions. J Appl Physiol 2000; 88: 1601–6
Williams RS, Harlan W. Effects of inhibition of mitochondrial protein synthesis in skeletal muscle. Am J Physiol 1987; 253: C866–71
Evans W. Functional and metabolic consequences of sarcopenia. J Nutr Suppl 1997; 127: 998S–1003S
Conley KE, Jubria SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol 2000; 526: 203–10
Rooyackers OE, Adey DB, Ades PA, et al. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A 1996; 93: 15364–9
Aspnes LE, Lee CM, Weindruch R, et al. Caloric restriction reduces fiber loss and mitochondrial abnormalities in aged rat muscle. FASEB J 1997; 11: 573–81
Lee CM, Aspnes LE, Chung SS, et al. Influences of caloric restriction on age-associated skeletal muscle fiber characteristics and mitochondrial changes in rats and mice. Ann N Y Acad Sci 1998; 854: 182–91
Lopez M, Van Zeeland N, Dahl D, et al. Cellular phenotypes of age-associated skeletal muscle mitochondrial abnormalities in rhesus monkeys. Mutat Res 2000; 452: 123–38
Wanagat J, Cao Z, Pathare P, et al. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fibre splitting and oxidative damage in sarcopenia. FASEB J 2001; 15: 322–32
Muller-Hocker J. Cytochrome C oxidase deficient fibres in the limb muscle and diaphragm of man without muscular disease: an age-related alteration. J Neurol Sci 1990, 21
Rifai Z, Welle S, Kamp C, et al. Ragged red fibers in normal aging and inflammatory myopathy. Ann Neurol 1995; 37: 24–9
Bresolin N, Moggio M, Bet L, et al. Progressive cytochrome C oxidase deficiency in a case of Kearns-Sayre syndrome: morphological, immunological and biochemical studies in muscle biopsies and autopsy tissues. Ann Neurol 1987; 21: 564–72
Bua EA, McKiernan SH, Wanagat J, et al. Mitochondrial abnormalities are more frequent in muscles undergoing sarcopenia. J Appl Physiol 2002; 92: 2617–24
Orlander J, Aniansson A. Effect of physical training on skeletal muscle metabolism and ultrastructure in 70- to 75-year-old men. Acta Physiol Scand 1980; 109: 149–54
Proctor DN, Sinning WE, Walro JM, et al. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol 1995; 78: 2033–8
Young JC, Chen M, Holloszy JO. Maintenance of the adaptation of skeletal muscle mitochondria to exercise in old rats. Med Sci Sports Exerc 1983; 15: 243–6
Sohal RS, Sohal BH. Hydrogen peroxide release by mitochondria increases during aging. Mech Ageing Dev 1991; 57: 187–202
Lezza AM, Pesce V, Cormio A, et al. Increased expression of mitochondrial transcription factor A and nuclear respiratory factor-1 in skeletal muscle from aged human subjects. FEBS Lett 2001; 501: 74–8
Pesce V, Cormio A, Fracasso F, et al. Age-related mitochondrial genotypic and phenotypic alterations in human skeletal muscle. Free Radie Biol Med 2001; 30: 1223–33
Cook SA, Sugden PH, Clerk A. Regulation of bcl-2 family proteins during development and in response to oxidative stress in cardiac myocytes: association with changes in mitochondrial membrane potential. Circ Res 1999; 85: 940–9
von Harsdorf R, Li PF, Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation 1999; 99: 2934–41
Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol 2001; 282: R519–27
Jubrias SA, Esselman PC, Price LB, et al. Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol 2001; 90: 1663–70
Taivassalo T, Shoubridge EA, Chen J, et al. Aerobic conditioning in patients with mitochondrial myopathies: physiological, biochemical, and genetic effects. Ann Neurol 2001; 50: 133–41
Taivassalo T, Fu K, Johns T, et al. Gene shifting: a novel therapy for mitochondrial myopathy. Hum Mol Genet 1999; 8: 1047–52
Acknowledgements
Work in the authors’ laboratory is funded by the Natural Science and Engineering Research Council (NSERC) of Canada and by the Canadian Institutes for Health Research. Peter J. Adhihetty is the recipient of a Heart and Stroke Foundation of Canada Doctoral Fellowship. Isabella Irrcher and Vladimir Ljubicic are recipients of NSERC Postgraduate Scholarships. David A. Hood is the holder of a Canada Research Chair in Cell Physiology. The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Irrcher, I., Adhihetty, P.J., Joseph, AM. et al. Regulation of Mitochondrial Biogenesis in Muscle by Endurance Exercise. Sports Med 33, 783–793 (2003). https://doi.org/10.2165/00007256-200333110-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00007256-200333110-00001