Summary
Abstract
Salmeterol/fluticasone propionate (Seretide®, Advair®, Viani®) administered using a multidose dry powder inhaler (Diskus®, Accuhaler®) is approved for use in the treatment of chronic obstructive pulmonary disease (COPD) in numerous countries.
Salmeterol/fluticasone propionate administered twice daily via dry powder inhaler is effective and generally well tolerated in patients with COPD. Although not associated with a statistically significant reduction in mortality versus placebo in the TORCH study (p = 0.052), salmeterol/fluticasone propionate reduced the rate of decline in lung function over the 3 years of the trial and was associated with lower exacerbation rates than the component monotherapies or placebo; other trials revealed clinically significant improvements in health status and dyspnoea scores with salmeterol/fluticasone propionate. Results of the INSPIRE trial suggest that salmeterol/fluticasone propionate is associated with a significantly lower mortality rate than tiotropium bromide monotherapy in patients with COPD; the two treatments had similar effects in terms of exacerbation rates and lung function. Thus, salmeterol/fluticasone propionate is an important option in the treatment of patients with COPD who are appropriate candidates for combination therapy with a long-acting bronchodilator and an inhaled corticosteroid.
Pharmacological Properties
Salmeterol is a highly selective β2-adrenoceptor agonist with, among other effects, long-acting bronchodilator activity, and fluticasone propionate is a corticosteroid with anti-inflammatory activity. Together, they appear to have additive, or even synergistic, effects. In patients with COPD, salmeterol/fluticasone propionate demonstrated beneficial effects on airway inflammation; fluticasone propionate alone may also have a beneficial effect on systemic inflammation in this patient group. The systemic effects of salmeterol/fluticasone propionate are similar to those of the individual agents, with no evidence of systemic pharmacodynamic interaction.
The pharmacokinetics of the salmeterol/fluticasone propionate combination product are similar to those of its individual components. Both salmeterol and fluticasone propionate are lipophilic and act locally in the lung, so plasma concentrations are not predictive of therapeutic effect. Hepatic clearance is predominantly responsible for the clearance of both salmeterol and fluticasone propionate, meaning that patients with hepatic disease who receive salmeterol/ fluticasone propionate should be closely monitored and caution is recommended when salmeterol/fluticasone propionate is co-administered with potent cytochrome P450 3A4 inhibitors.
Therapeutic Efficacy
A number of well designed trials have compared the efficacy of salmeterol/ fluticasone propionate 50μg/250μg or 50μg/500μg administered twice daily via dry powder inhaler with that of various comparators in patients with COPD.
Several randomised, double-blind, multicentre studies of 24 weeks to 3 years’ duration have compared twice-daily salmeterol/fluticasone propionate 50μg/ 250μg or 50μg/500μg with the component monotherapies and/or placebo in patients with COPD. Results of the 3-year TORCH study did not reveal a statistically significant difference in mortality between salmeterol/fluticasone propionate and either placebo or salmeterol alone, although there was a significantly lower probability of death with salmeterol/fluticasone propionate than with fluticasone propionate alone. In two studies comparing salmeterol/fluticasone propionate with the component monotherapies, including the TORCH study, significantly fewer moderate to severe exacerbations occurred with salmeterol/ fluticasone propionate than with the component monotherapies. The results of the 3-year TORCH study suggest that long-term therapy with salmeterol/fluticasone propionate in COPD reduced the rate of decline in lung function. In all studies, lung function improved to a significantly greater extent with salmeterol/fluticasone propionate than with component monotherapies or placebo. In addition, some trials revealed clinically significant improvements in health status and dyspnoea scores with salmeterol/fluticasone propionate.
Twice-daily salmeterol/fluticasone propionate 50μg/500μg had a similar effect to once-daily tiotropium bromide 18μg in terms of healthcare utilisation and symptom-based exacerbation rates and lung function in patients with severe COPD, according to the results of the 2-year, randomised, double-blind, multicentre INSPIRE trial. However, the relative probability of all-cause mortality was significantly lower with salmeterol/fluticasone propionate than with tiotropium bromide.
Twice-daily salmeterol/fluticasone propionate 50μg/250μg was more effective than four-times-daily salbutamol/ipratropium bromide 206μg/36μg, administered via metered-dose inhaler, in the treatment of COPD, according to the results of two randomised, double-blind, multicentre, 8-week studies. The change from baseline in morning predose forced expiratory volume in 1 second (FEV1) significantly favoured patients receiving salmeterol/fluticasone propionate in both studies, as did the 6-hour area under the FEV1 curve, morning peak expiratory flow (PEF), the percentage of symptom-free nights, the transitional dyspnoea index focal score and the overall combined daytime symptom score.
There was no significant difference between patients receiving salmeterol/ fluticasone propionate 50μg/500μg twice daily or fluticasone propionate 500μg plus oral sustained-release theophylline, both administered twice daily, in the change from baseline in FEV1 after 4 months of treatment in a randomised nonblind study in patients with COPD. However, the reductions from baseline in the visual analogue scale score for dyspnoea and rescue medication use significantly favoured salmeterol/fluticasone propionate.
Administering twice-daily salmeterol/fluticasone propionate 50μg/500μg in combination with once-daily tiotropium bromide 18μg did not confer additional enefit to patients with COPD in terms of exacerbation rates (vs once-daily tiotropium bromide 18μg alone), although some benefits were seen in terms of hospitalisation rates (vs once-daily tiotropium bromide 18μg alone), lung function (vs twice-daily salmeterol/fluticasone propionate 50μg/500μg alone or once-daily tiotropium bromide 18μg alone) and health status (vs once-daily tiotropium bromide 18μg alone), according to the results of three randomised, double-blind trials.
Withdrawing fluticasone propionate resulted in a significant, albeit small, decline in FEV1 in patients with COPD who had been receiving salmeterol/ fluticasone propionate 50μg/500μg twice daily for 3 months according to the results of a randomised, double-blind, multicentre trial. Moreover, significantly greater reductions from baseline in FEV1: forced vital capacity and PEF were seen with salmeterol alone versus salmeterol/fluticasone propionate, although results were mixed in terms of other endpoints.
Tolerability
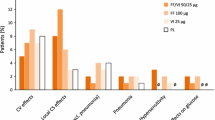
In general, adverse events occurring with salmeterol/fluticasone propionate were those that would be expected with the component drugs. In the TORCH study, the most commonly occurring adverse events in salmeterol/fluticasone propionate recipients included COPD exacerbation, upper respiratory tract infection, nasopharyngitis, pneumonia, candidiasis, bronchitis, headache, back pain, sinusitis, cough and hypertension.
In the TORCH and INSPIRE studies, there were no significant between-group differences in fracture incidence. An additional safety analysis conducted in a subgroup of patients from the TORCH trial revealed that there were no significant between-group differences in the change from baseline in bone mineral density of the hip or lumbar spine. In the TORCH study, there was no significant difference between patients receiving salmeterol/fluticasone propionate, salmeterol alone, fluticasone propionate alone or placebo in the incidence of eye disorders, including the development of cataracts. The incidence of pneumonia was significantly higher with salmeterol/fluticasone propionate or fluticasone propionate alone than with placebo in the TORCH study, and with salmeterol/fluticasone propionate than with tiotropium bromide in the INSPIRE study.
Pharmacoeconomic Considerations
Salmeterol/fluticasone propionate was a cost-effective option in the treatment of COPD, according to the results of a pharmacoeconomic analysis conducted in conjunction with the TORCH study; the analysis was conducted from the perspective of the UK National Health Service. The incremental cost-effectiveness ratio (ICER) for salmeterol/fluticasone propionate versus placebo was £17 000 (95% CI 8100, 41 700) per quality-adjusted life-year (QALY) gained (year of costing not stated). Results of a modelling study conducted from the perspective of the Canadian Ministry of Health also found salmeterol/fluticasone propionate to be a potentially cost-effective option in the treatment of patients with poorly reversible COPD and frequent exacerbations. The ICER for salmeterol/fluticasone propionate versus placebo was $Can74 887 per QALY gained (2002 values).






Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechansims. Eur Respir J 2003 Oct; 22(4): 672–88
Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006; 27(2): 397–412
Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007 Sep 15; 176(6): 532–55
Rodríguez-Roisin R. The airway pathophysiology of COPD: implications for treatment. COPD 2005 Jun; 2: 253–62
Agusti AGN. COPD, a multicomponent disease: implications for management. Respir Med 2005 Jun; 99(6): 670–82
Dransfield MT, Bailey WC. Fluticasone propionate/salmeterol for the treatment of chronic-obstructive pulmonary disease. Expert Opin Pharmacother 2004 Aug; 5(8): 1815–26
Kirby S, Falcoz C, Daniel MJ, et al. Salmeterol and fluticasone propionate given as a combination: lack of systemic pharmacodynamic and pharmacokinetic interactions. Eur J Clin Pharmacol 2001; 56: 781–91
Tay HL, Armoogum N, Tan LKS. Nasal mucociliary clearance and salmeterol. Clin Otolaryngol 1997; 22: 68–70
Lindberg A, Szalai Z, Pullerits T, et al. Fast onset of effect of budesonide/formoterol versus salmeterol/fluticasone and salbutamol in patients with chronic obstructive pulmonary disease and reversible airway obstruction. Respirology 2007 Sep; 12(5): 732–9
Barnes NC, Qiu Y-S, Pavord ID, et al. Antiinflammatory effects of salmeterol/fluticasone propionate in chronic obstructive lung disease. Am J Respir Crit Care Med 2006 Apr 1; 173(7): 736–43
Bourbeau J, Christodoulopoulos P, Maltais F, et al. Effect of salmeterol/fluticasone propionate on airway inflammation in COPD: a randomized controlled trial. Thorax. Epub 2007 Jun 8
Sin DD, Lacy P, York E, et al. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004 Oct 1; 170(7): 760–5
Di Marco F, Milic-Emili J, Boveri B, et al. Effect of inhaled bronchodilators on inspiratory capacity and dyspnoea at rest in COPD. Eur Respir J 2003; 21: 86–94
Cazzola M, Matera MG, Santangelo G, et al. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med 1995 May; 89: 357–62
Cazzola M, Santus P, Di Marco F, et al. Bronchodilator effect of an inhaled combination therapy with salmeterol + fluticasone and formoterol + budesonide in patients with COPD. Respir Med 2003 May; 97(5): 453–7
Cazzola M, Santangelo G, Piccolo A. Effect of salmeterol and formoterol in patients with chronic obstructive pulmonary disease. Pulm Pharmacol 1994 Apr; 7(2): 103–7
GlaxoSmithKline. Advair Diskus® 100/50, 250/50, 500/50 prescribing information [online]. Available from URL: http://www.gsk.com/products/advair_us.htm [Accessed 2007 Jul 30]
Buchwald A, Hochhaus G. Pharmacokinetic and pharmacodynamic aspects of salmeterol therapy. Int J Clin Pharmacol Ther 1998 Dec; 36(12): 652–60
Sin DD, Man SFP. Corticosteroids and adrenoceptor agonists: the compliments for combination therapy in chronic airways diseases. Eur J Pharmacol 2006 Mar 8; 533(1–3): 28–35
Sekut L, Champion BR, Page K, et al. Anti-inflammatory activity of salmeterol: down-regulation of cytokine production. Clin Exp Immunol 1995; 99(3): 461–6
Pang L, Knox AJ. Regulation of TNF-α-induced eotaxin release from cultured human airway smooth muscle cells by β2-agonists and corticosteroids. FASEB J 2001 Jan; 15: 261–9
Proud D, Reynolds CJ, Lichtenstein LM, et al. Intranasal salmeterol inhibits allergen-induced vascular permeability but not mast cell activation or cellular infiltration. Clin Exp Allergy 1998 Jul; 28(7): 868–75
Kanthakumar K, Cundell DR, Johnson M, et al. Effect of salmeterol on human nasal epithelial cell ciliary beating: inhibition of the ciliotoxin, pyocyanin. Br J Pharmacol 1994; 112: 493–8
Piatti G, Ambrosetti U, Santus P, et al. Effects of salmeterol on cilia and mucus in COPD and pneumonia patients. Pharmacol Res 2005 Feb; 51(2): 165–8
Sin DD, Man SFP. Do chronic inhaled steroids alone or in combination with a bronchodilator prolong life in chronic obstructive pulmonary disease patients? Curr Opin Pulm Med 2007 Mar; 13(2): 90–7
Mollmann H, Wagner M, Krishnaswami S, et al. Single-dose and steady-state pharmacokinetic and pharmacodynamic evaluation of therapeutically equivalent doses of inhaled fluticasone propionate and budesonide, given as Diskus or Turbohaler dry-powder inhalers to healthy subjects. J Clin Pharmacol 2001; 41: 1329–38
Thorsson L, Edsbäcker S, Källen A, et al. Pharmacokinetics and systemic activity of fluticasone via Diskus® and pMDI, and of budesonide via Turbuhaler®. Br J Clin Pharmacol 2001; 52: 529–38
Pang L, Knox AJ. Synergistic inhibition by β2-agonists and corticosteroids on tumor necrosis factor (TNF)-α-induced interleukin-8 release from cultured human airway smooth-muscle cells. Am J Respir Cell Mol Biol 2000; 23(1): 79–85
Pace E, Gagliardo R, Melis M, et al. Synergistic effects of fluticasone propionate and salmeterol on in vitro T-cell activation and apoptosis in asthma. J Allergy Clin Immunol 2004 Nov; 114(5): 1216–23
Wallin A, Sue-Chu M, Bjermer L, et al. Effect of inhaled fluticasone with and without salmeterol on airway inflammation in asthma. J Allergy Clin Immunol 2003 Jul; 112(1): 72–8
Baraniuk JN, Ali M, Brody D, et al. Glucocorticoids induce β2-adrenergic receptor function in human nasal mucosa. Am J Respir Crit Care Med 1997 Feb; 155(2): 704–10
Kalavantavanich K, Schramm CM. Dexamethasone potentiates high-affinity β-agonist binding and GsCα protein expression in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2000 May; 278(5): L1101–6
Mak JCW, Nishikawa M, Shirasaki H, et al. Protective effects of a glucocorticoid on downregulation of pulmonary β2-adrenergic receptors in vivo. J Clin Invest 1995; 96: 99–106
Eickelberg O, Roth M, Lörx R, et al. Ligand-independent activation of the glucocorticoid receptor by β2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J Biol Chem 1999 Jan 8; 274(2): 1005–10
Usmani OS, Ito K, Maneechotesuwan K, et al. Glucocorticoid receptor nuclear translocation in airway cells after inhaled combination therapy. Am J Respir Crit Care Med 2005 Sep 15; 172(6): 704–12
Haque RA, Torrego A, Essilfie-Quaye S, et al. Effect of salmeterol and fluticasone on glucocorticoid receptor translocation in sputum macrophages and peripheral blood mononuclear cells from patients with chronic obstructive pulmonary disease [abstract]. Proc Am Thoracic Soc 2006 Apr; 3 Suppl.: A848
Vestbo J, Pauwels R, Anderson JA, et al. Early onset of effect of salmeterol and fluticasone propionate in chronic obstructive pulmonary disease. Thorax 2005 Apr; 60(4): 301–4
Dowling RB, Johnson M, Cole PJ, et al. Effect of salmeterol on Haemophilus influenzae infection of respiratory mucosa in vitro. Eur Respir J 1998; 11: 86–90
Dowling RB, Johnson M, Cole PJ, et al. Effect of fluticasone propionate and salmeterol on Pseudomonas aeruginosa infection of the respiratory mucosa in vitro. Eur Respir J 1999; 14: 363–9
Mehta RS, Kathman SJ, Daley-Yates PT, et al. Pharmacokinetics and pharmacodynamics in COPD patients following long-term twice-daily treatment with salmeterol/fluticasone propionate 50/500mg and the individual components [abstract]. Am J Respir Crit Care Med 2007 Apr; 175 Suppl.: 127
Calverley PMA, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007 Feb 22; 356(8): 775–89
Singh SD, Whale C, Hatton A, et al. Comparison of the pharmacokinetics (PK) of inhaled fluticasone propionate (FP) in patients with chronic obstructive pulmonary disease (COPD) and healthy subjects [abstract]. Am J Rep Crit Care Med 2001 Apr; 163 (5 Suppl. Pt 2): A281
Daley-Yates PT, Harker AJ, Taylor S, et al. Plasma protein binding (PPB) of corticosteroids (CS): reappraisal of its significance in systemic pharmacological activity [abstract no. 13]. J Allergy Clin Immunol 2005 Feb; 115 (2 Suppl. 1): S4
Cazzola M, Testi R, Matera MG. Clinical pharmacokinetics of salmeterol. Clin Pharmacokinet 2002; 41(1): 19–30
Allen & Hanburys. Seretide 100, 250, 500 Accuhaler [online]. Available from URL: http://emc.medicines.org.uk [Accessed 2007 Sep 26]
Manchee GR, Barrow A, Kulkarni S, et al. Disposition of salmeterol xinafoate in laboratory animals and humans. Drug Metab Dispos 1993; 21(6): 1022–8
Hanania NA, Darken P, Horstman D, et al. The efficacy and safety of fluticasone propionate (250 μg)/salmeterol (50 μg) combined in the Diskus inhaler for the treatment of COPD. Chest 2003 Sep; 124(3): 834–43
Kardos P, Wencker M, Glaab T, et al. Impact of salmeterol/ fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007 Jan 15; 175(2): 144–9
Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003 Feb 8; 361(9356): 449–56
Mahler DA, Wire P, Horstman D, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002 Oct 15; 166(8): 1084–91
Celli B, Ferguson GT, Anderson JA, et al. Salmeterol/fluticasone propionate (SFC) improves lung function and reduces the rate of decline over three years in the TORCH survival study [abstract]. Am J Respir Crit Care Med 2007 Apr; 175 Suppl.: A763
Wedzicha JA, Calverley PMA, Seemungal TA, et al. The prevention of COPD exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. Epub 2007 Oct 4
Make B, Hanania NA, ZuWallack R, et al. The efficacy and safety of inhaled fluticasone propionate/salmeterol and ipratropium/albuterol for the treatment of chronic obstructive pulmonary disease: an eight-week, multicenter, randomized, double-blind, double-dummy, parallel-group study. Clin Ther 2005 May; 27(5): 531–42
Donohue JF, Kalberg C, Emmett A, et al. A short-term comparison of fluticasone propionate/salmeterol with ipratropium bromide/albuterol for the treatment of COPD. Treat Respir Med 2004; 3(3): 173–81
Cazzola M, Noschese P, Centanni S, et al. Salmeterol/fluticasone propionate in a single inhaler device versus theophylline + fluticasone proionate in patients with COPD. Pulm Pharmacol Ther 2004; 17: 141–5
Singh D, Brooks J, Hagan G, et al. Superiority of the ‘triple’ therapy of salmeterol/fluticasone propionate (SFC) and tiotropium (TIO) vs. individual components in COPD [abstract]. 17th Annual Congress of the European Respiratory Society; 2007 Sep 15–19; Stockholm
Cazzola M, Andò F, Santus P, et al. A pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe-to-very severe COPD. Pulm Pharmacol Ther 2007; 20(5): 556–61
Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2007 Apr 17; 146(8): 545–55
Wouters EFM, Postma DS, Fokkens B, et al. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trial. Thorax 2005 Jun; 60(6): 480–7
Lee TA, Weiss KB. Fracture risk associated with inhaled corticosteroid use in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004 Apr 1; 169(7): 855–9
de Vries F, van Staa TP, Bracke MSGM, et al. Severity of obstructive airway disease and risk of osteoporotic fracture. Eur Respir J 2005; 25: 879–84
Glick H, Briggs A, Lozano-Ortega G, et al. Is treatment with ICS/LABA combination good value for money in COPD? Evidence from the TORCH study [abstract]. Am J Respir Crit Care Med 2007 Apr; 175 Suppl.: A763
Spencer M, Briggs AH, Grossman RF, et al. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics 2005; 23(6): 619–37
Halpin DMG, Miravitlles M. Chronic obstructive pulmonary disease: the disease and its burden to society. Proc Am Thoracic Soc 2006; 3(7): 619–23
American Thoracic Society and European Respiratory Society. Standards for the diagnosis and treatment of patients with chronic obstructive pulmonary disease [online]. Available from URL: http://www.thoracic.org/COPD/default.asp [Accessed 2007 Jul 27]
Calverley PMA, Scott S. Is airway inflammation in chronic obstructive pulmonary disease (COPD) a risk factor for cardiovascular events? COPD 2006 Dec; 3(4): 233–42
Agustí AGN, Noguera A, Sauleda J, et al. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J 2003; 21: 347–60
ClinicalTrials.gov. Can Advair and Flovent reduce systemic inflammation related to chronic obstructive pulmonary disease (COPD)? A multi-center randomized controlled trial [online]. Available from URL: http://clinicaltrials.gov [Accessed 2007 Aug 16]
Soriano JB, Vestbo J, Pride NB, et al. Survival in COPD patients after regular use of fluticasone propionate and salmeterol in general practice. Eur Respir J 2002 Oct; 20(4): 819–25
Soriano JB, Kiri VA, Pride NB, et al. Inhaled corticosteroids with/without long-acting β-agonists reduce the risk of rehospitalization and death in COPD patients. Am J Respir Med 2003; 2(1): 67–74
Mapel DW, Nelson LS, Lydick E, et al. Survival among COPD patients using fluticasone/salmeterol in combination versus other inhaled steroids and bronchodilators alone. COPD 2007 Jun; 4(2): 127–34
Mapel DW, Hurley JS, Roblin D, et al. Survival of COPD patients using inhaled corticosteroids and long-acting beta agonists. Respir Med 2006; 100: 595–609
Rabe KF. Treating COPD; the TORCH trial, p values, and the dodo. N Engl J Med 2007 Feb 22; 356(8): 851–4
Donohue JF. Combination therapy for chronic obstructive pulmonary disease: clinical aspects. Proc Am Thorac Soc 2005; 2(4): 272–81
Meinke L, Chitkara R, Krishna G. Advances in the management of chronic obstructive pulmonary disease. Expert Opin Pharmacother 2007; 8(1): 23–37
GlaxoSmithKline. GSK receives decision from FDA on Advair 500/50 for COPD [media release]. Available from URL: http://www.gsk.com [Accessed 2007 Aug 16]
Atkins PJ. Dry powder inhalers: an overview. Respir Care 2005 Oct; 50(10): 1304–12
Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J 2003; 21: 74–81
Calverley PM, Boonsawat W, Cseke Z, et al. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J 2003; 22: 912–9
Decramer M, Ferguson G. Clinical safety of long-acting β2-agonist and inhaled corticosteroid combination therapy in COPD. COPD 2006 Sep; 3: 163–71
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: J. Bourbeau, Respiratory Epidemiology and Clinical Research Unit, McGill University Health Center, Montreal, Quebec, Canada; M. Cazzola, Unit of Respiratory Diseases, Department of Internal Medicine, University of Rome ‘Tor Vergata’, Rome, Italy; N.A. Hanania, Section of Pulmonary and Critical Care Medicine, Asthma Clinical Research Center, Baylor College of Medicine, Houston, Texas, USA; P.K. Jeffery, Lung Pathology, Royal Brompton Hospital, London, England; P. Santus, Institute of Lung Disease, Respiratory Unit, San Paolo Hospital, University of Milan, Milan, Italy; E.F.M. Wouters, Department of Respiratory Medicine, University Hospital Maastricht, Maastricht, The Netherlands.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘salmeterol/fluticasone’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Wolters Kluwer Health | Adis). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were ‘salmeterol/fluticasone’ and (‘chronic obstructive pulmonary disease’ or ‘COPD’). Searches were last updated 15 October 2007.
Selection: Studies in patients with chronic obstructive pulmonary disease who received salmeterol/fluticasone propionate. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Salmeterol, fluticasone propionate, chronic obstructive pulmonary disease, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Keating, G.M., McCormack, P.L. Salmeterol/Fluticasone Propionate. Drugs 67, 2383–2405 (2007). https://doi.org/10.2165/00003495-200767160-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200767160-00006