Abstract
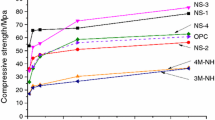
The influences of aluminum sulfate (AS) introduction and dosage on setting time, hydration heat evolution, hydration product type and pore structure of Portland cement were studied, and the influence of AS on concrete strength was investigated also. The results indicate that AS can effectively accelerate setting time of Portland cement and enhance concrete at early age (1 day) strength. AS can promote hydration process of calcium aluminate but inhibit that of calcium silicate. The effect of AS on hydration process becomes more significant along with the increased dosage; and the introduction of AS can promote the formation of AFt. The research results of this paper favor the opinion of the existence of AFt precursor; and the AFt precursor is amorphous AFm which could not be identified by XRD. With anhydrite as setting regulator, the amorphous AFm retention time is prolonged, and the endothermal peaks produced by amorphous AFm during DSC–MS measurement correspond to 80–160 and 830–910 °C, losing H2O and SO2 respectively.








Similar content being viewed by others
References
Cheung J, Jeknavorian A, Roberts L et al (2011) Impact of admixtures on the hydration kinetics of Portland cement. Cem Concr Res 41(12):1289–1309
Prudencio LR Jr (1998) Accelerating admixtures for shotcrete. Cem Concr Compos 20(2):213–219
Heikal M (2004) Effect of calcium formate as an accelerator on the physicochemical and mechanical properties of pozzolanic cement pastes. Cem Concr Res 34(6):1051–1056
Şahmaran M, Özkan N, Keskin SB et al (2008) Evaluation of natural zeolite as a viscosity-modifying agent for cement-based grouts. Cem Concr Res 38(7):930–937
Janotka I, Puertas F, Palacios M et al (2010) Metakaolin sand–blended-cement pastes: rheology, hydration process and mechanical properties. Constr Build Mater 24(5):791–802
Pourchet S, Regnaud L, Perez JP et al (2009) Early C3A hydration in the presence of different kinds of calcium sulfate. Cem Concr Res 39(11):989–996
Paglia C, Wombacher F, Böhni H (2001) The influence of alkali-free and alkaline shotcrete accelerators within cement systems: I. characterization of the setting behavior. Cem Concr Res 31(6):913–918
DiNoia TP, Saandberg PJ (2004) Alkali free shotcrete accelerator interactions with cement and admixture, shotcrete: more engineering developments. Taylor & Francis, London, pp 137–144
Maltese C, Pistolesi C, Bravo A et al (2007) Effects of setting regulators on the efficiency of an inorganic acid based alkali-free accelerator reacting with a Portland cement. Cem Concr Res 37(4):528–536
Taylor HFW (1997) Cement chemistry, 2nd edn. Thomas Telford, London, pp 150–225
Bullard JW, Jennings HM, Livingston RA et al (2011) Mechanisms of cement hydration. Cem Concr Res 41(12):1208–1223
Quennoz A, Scrivener KL (2012) Hydration of C3A–gypsum systems. Cem Concr Res 42(7):1032–1041
Bensted J (1982) Effects of the clinker–gypsum grinding temperature upon early hydration of Portland cement. Cem Concr Res 12(3):341–348
Minard H, Garrault S, Regnaud L et al (2007) Mechanisms and parameters controlling the tricalcium aluminate reactivity in the presence of gypsum. Cem Concr Res 37(10):1418–1426
Bhatty JI (1991) A review of the application of thermal analysis to cement-admixture systems. Thermochim Acta 189(2):313–350
Ramachandran VS (1988) Thermal analyses of cement components hydrated in the presence of calcium carbonate. Thermochim Acta 127:385–394
Plowman C, Cabrera JG (1984) Mechanism and kinetics of hydration of C3A and C4AF. Extracted from cement. Cem Concr Res 14(2):238–248
Abdelrazig BEI, Bonner DG, Nowell DV et al (1989) Estimation of the degree of hydration in modified ordinary Portland cement pastes by differential scanning calorimetry. Thermochim Acta 145:203–217
Ramachandran VS (1972) Elucidation of the role of chemical admixtures in hydrating cements by DTA technique. Thermochim Acta 4(3):343–366
Kovler K (1998) Setting and hardening of gypsum–Portland cement–silica fume blends, part 2: early strength, DTA, XRD, and SEM observations. Cem Concr Res 28(4):523–531
Odler I, Wonnemann R (1983) Effect of alkalies on Portland cement hydration: I. Alkali oxides incorporated into the crystalline lattice of clinker minerals. Cem Concr Res 13(4):477–482
Pane I, Hansen W (2005) Investigation of blended cement hydration by isothermal calorimetry and thermal analysis. Cem Concr Res 35(6):1155–1164
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, J., Wang, K., Wang, Y. et al. Study of aluminum sulfate and anhydrite on cement hydration process. Mater Struct 49, 1105–1114 (2016). https://doi.org/10.1617/s11527-015-0561-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1617/s11527-015-0561-2