Abstract
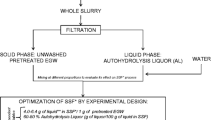
Alkaline detoxification strongly improves the fermentability of dilute-acid hydrolysates in the production of bioethanol from lignocellulose with Saccharomyces cerevisiae. New experiments were performed with NH4OH and NaOH to define optimal conditions for detoxification and make a comparison with Ca(OH)2 treatment feasible. As too harsh conditions lead to sugar degradation, the detoxification treatments were evaluated through the balanced ethanol yield, which takes both the ethanol production and the loss of fermentable sugars into account. The optimization treatments were performed as factorial experiments with 3-h duration and varying pH and temperature. Optimal conditions were found roughly in an area around pH 9.0/60°C for NH4OH treatment and in a narrow area stretching from pH 9.0/80°C to pH 12.0/30°C for NaOH treatment. By optimizing treatment with NH4OH, NaOH, and Ca(OH)2, it was possible to find conditions that resulted in a fermentability that was equal or better than that of a reference fermentation of a synthetic sugar solution without inhibitors, regardless of the type of alkali used. The considerable difference in the amount of precipitate generated after treatment with different types of alkali appears critical for industrial implementation.
Similar content being viewed by others
References
Larsson, S., Reimann, A., Nilvebrant, N.-O., and Jönsson, L. J. (1999), Appl. Biochem. Biotechnol. 77–79, 91–103.
Martinez, A., Rodriguez, M. E., York, S. W., Preston, J. F., and Ingram, L.-O. (2000), Biotechnol. Bioeng. 69, 526–536.
Martinez, A., Rodriguez, M. E., Wells, M. L., York, S. W., Preston, J. F., and Ingram, L. O. (2001), Biotechnol. Prog. 17, 287–93.
Persson, P., Andersson, J., Gorton, L., Larsson, S., Nilvebrant, N.-O., and Jönsson, L. J. (2002), J. Agric. Food Chem. 50, 5318–5325.
Millati, R., Niklasson, C., and Taherzadeh, M. J. (2002), Process Biochem. 38, 515–522.
Sárvári Horváth, I., Sjöde, A., Alriksson, B., Jönsson, L. J., and Nilvebrant, N.-O. (2005), Appl. Biochem. Biotechnol. 121–124, 1031–1044.
Nigam, J. N. (2001), J. Biotechnol. 87, 17–27.
Alriksson, B., Sárvári Horváth, I., Sjöde, A., Nilvebrant, N.-O., and Jönsson, L. J. (2005), Appl. Biochem. Biotechnol. 121–124, 911–922.
Nilvebrant, N.-O., Reimann, A., Larsson, S., and Jönsson, L. J. (2001), Appl. Biochem. Biotechnol. 91–93, 35–49.
Nilvebrant, N.-O., Persson, P., Reimann, A., de Sousa, F., Gorton, L., and Jönsson, L. J. (2003), Appl. Biochem. Biotechnol. 105–108, 615–628.
Sárvári Horváth, I., Sjöde, A., Nilvebrant, N.-O., Zagorodni, A., and Jönsson, L. J. (2004), Appl. Biochem. Biotechnol. 114, 525–538.
Singleton, V. L., Orhofer, R., and Lamuela-Raventos, R. M. (1999), Methods Enzymol. 299, 152–178.
Larsson, S., Palmqvist, E., Hahn-Hägerdal, B., et al. (1999), Enzyme Microb. Technol. 24, 151–159.
Verduyn, C., Postma, E., Scheffer, W. A., and van Dijken, J. P. (1992) Yeast 8, 501–517.
Taherzadeh, M. J., Niklasson, C., and Lidén, G. (1997), Chem. Eng. Sci. 52, 2653–2659
Lawford, H. G. and Rousseau J.D. (2003), Appl. Biochem. Biotechnol. 106, 457–470.
de Bruijn, J. M., Kieboom, A. P. G., van Bekkum, H., and van der Poel, P. W. (1986) Sugar Technol. Rev. 13, 21–52.
Van Zyl, C., Prior, B. A., and Du Preez, J. C. (1988), Appl. Biochem. Biotechnol. 17, 357–369.
Taherzadeh, M. J., Eklund, R., Gustafsson, L. C., and Lidén, G. (1997) Ind. Eng. Chem. Res. 36, 4659–4665.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alriksson, B., Sjöde, A., Nilvebrant, NO. et al. Optimal conditions for alkaline detoxification of dilute-acid lignocellulose hydrolysates. Appl Biochem Biotechnol 130, 599–611 (2006). https://doi.org/10.1385/ABAB:130:1:599
Issue Date:
DOI: https://doi.org/10.1385/ABAB:130:1:599