Abstract
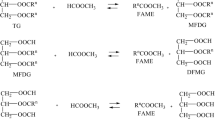
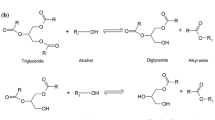
Biodiesel fuel was prepared by a two-step reaction: hydrolysis and methyl esterification. Hydrolysis was carried out at a subcritical state of water to obtain fatty acids from triglycerides of rapeseed oil, while the methyl esterification of the hydrolyzed products of triglycerides was treated near the supercritical methanol condition to achieve fatty acid methyl esters. Consequently, the two-step preparation was found to convert rapessed oil to fatty acid methyl esters in considerably shorter reaction time and milder reaction condition than the direct supercritical methanol treatment. The optimum reaction condition in this two-step preparation was 270°C and 20 min for hydrolysis and methyl esterification, respectively. Variables affecting the yields in hydrolysis and methyl esterification are discussed.
Similar content being viewed by others
References
Fukuda, H., Kondo, A., and Noda, H. (2001), J. Biosci. Bioeng. 92, 405–416.
Haas, M. J. and Bloomer, S. (2000), JAOCS 77, 373–379.
Frohlich, A., Rice, B., and Vicente, G. (2001), in Proceedings of 1st World Conference on Biomass for Energy and Industry, James & James (Science Publishers), London, UK, pp. 695–697.
Boocock, D. G. B. (2002), in Proceedings of Kyoto University International Symposium on Post-Petrofuels in the, 21 st Century: the Prospects in the Future of Biomass Energy, September 3–4, 2002, Montreal, Canada, pp. 171–177.
Muckerheide, V. J. (1952), JAOCS 28, 490–495.
Mills, V. and McClain, H. K. (1949), Ind. Eng. Chem. 26, 1982–1985.
Reinish, M. D. (1956), JAOCS 33, 516–520.
Albasi, C., Bertrand, N., and Riba, J. P. (1999), Bioprocess Eng. 10, 77–81.
Holliday, R. L., King, J. W. and List, G. R. (1997), Ind. Eng. Chem. Res. 36, 832–935.
Kusdiana, D. and Saka, S. (2001), J. Chem. Eng. Japan, 34, 383–387.
Warabi, Y., Kusdiana, D., and Saka, S. (2004), Bioresour. Technol. 91, 283–287.
Saka, S. and Kusdiana, D. (2001), Fuel 80, 225–231.
Kusdiana, D. and Saka, S. (2001), Fuel 80, 693–698.
Kusdiana, D., Minami, E., Ehara, K., and Saka, S. (2002), in Proceedings of the 12 th European Biomass Conference, James & James (Science Publishers), London, UK, pp. 789–792.
Kusdiana, D. and Saka, S. (2004), Bioresour. Technol. 91, 289–295.
Broll, D., Kaul, C., Kramer, A., Krammer, P., Richter, T., Jung, M., Vogel, H., and Zehner, P. (1999), Angew. Chem. Int. Ed. 38, 2998–3014.
Rudan-Tasic, D. and Klofutar, C. (1999), Acta Chim. Slov. 46, 511–521.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kusdiana, D., Saka, S. Two-step preparation for catalyst-free biodiesel fuel production. Appl Biochem Biotechnol 115, 781–791 (2004). https://doi.org/10.1385/ABAB:115:1-3:0781
Issue Date:
DOI: https://doi.org/10.1385/ABAB:115:1-3:0781