Abstract
Background
Little is known about changes in body composition that may occur during neoadjuvant therapy for pancreatic cancer. This study was designed to characterize these changes and their potential relationships with therapeutic outcomes.
Methods
The study population consisted of patients with potentially resectable pancreatic cancer treated on a phase II trial of neoadjuvant chemotherapy and chemoradiation. Skeletal muscle and adipose tissue compartments were measured before and after administration of neoadjuvant therapy using SliceOMatic software (TomoVision, 2012) and protocol-mandated CT scans. Sarcopenia was defined using gender-adjusted norms.
Results
Among 89 eligible patients, 46 (52 %) patients met anthropometric criteria for sarcopenia prior to the initiation of neoadjuvant therapy. Further depletion of skeletal muscle, visceral adipose tissue, and subcutaneous adipose tissue occurred during neoadjuvant therapy, but these losses did not preclude the performance of potentially curative surgery. Degree of skeletal muscle loss correlated with disease-free survival while visceral adipose loss was associated with overall and progression-free survival. However, completion of all therapy, including pancreatectomy, was the only independently significant predictor of outcome in a multivariate analysis of overall survival.
Discussion
These data suggest that body composition analysis of standard CT images may provide clinically relevant information for patients with potentially resectable pancreatic cancer who receive neoadjuvant therapy. Anthropometric changes must be considered in the design of preoperative therapy regimens, and further efforts should focus on maintenance of muscle and visceral adipose tissue in the preoperative setting.

Similar content being viewed by others
References
Rosenberg IH, Roubenoff R. Stalking sarcopenia. Ann Intern Med. 1995;123:727–8.
Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:1478–86.
Dalal S, Hui D, Bidaut L, et al. Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: a pilot study. J Pain Sympt Manag. 2012;44:181–91.
Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15:6973–9.
Di Sebastiano KM, Yang L, Zbuk K, et al. Accelerated muscle and adipose tissue loss may predict survival in pancreatic cancer patients: the relationship with diabetes and anaemia. Br J Nutr. 2013;109:302–12.
Jacquillat C, Baillet F, Auclerc G, et al. Neoadjuvant chemotherapy of breast cancer. Drugs Exp Clin Res. 1986;12:147–52.
Scholl SM, Asselain B, Palangie T, et al. Neoadjuvant chemotherapy in operable breast cancer. Eur J Cancer. 1991;27:1668–71.
Mehta VK, Poen J, Ford J, et al. Radiotherapy, concomitant protracted-venous-infusion 5-fluorouracil, and surgery for ultrasound-staged T3 or T4 rectal cancer. Dis Colon Rectum. 2001;44:52–8.
Mohiuddin M, Winter K, Mitchell E, et al. Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group Trial 0012. J Clin Oncol. 2006;24:650–5.
Ancona E, Ruol A, Santi S, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer. 2001;91:2165–74.
Walsh TN, Grennell M, Mansoor S, Kelly A. Neoadjuvant treatment of advanced stage esophageal adenocarcinoma increases survival. Dis Esophagus. 2002;15:121–4.
Del Fabbro E, Parsons H, Warneke CL, et al. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist. 2012;17:1240–5.
Awad S, Tan BH, Cui H, et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr (Edinburgh). 2012;31:74–7.
Clark W, Siegel EM, Chen YA, et al. Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg. 2013;216:1070–81.
Tempero MA, Malafa MP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2014. J Natl Comp Cancer Netw. 2014;12:1083–93.
Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–95.
Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–502.
Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935–49; discussion 949–50.
Evans DB, Lee JE, Tamm EP, Pisters PWT. Pancreaticoduodenectomy (Whipple operation) and total pancreatectomy for cancer. In: Fischer J, editor Master of surgery. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007:1299–317.
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006.
Mehta CR, Patel NR. A network algorithm for performing Fisher’s exact test in r x c contingency tables. J Am Stat Assoc. 1983;78:427–34.
Kaplan EL, Meier P. Nonparametric estimator from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220.
Lausen B, Sauerbrei W, Schumacher V. Classification and regression trees (CART) used for the exploration of prognostic factors measured on different scales. Contr Stat. 1994:483–96.
Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80:271–8.
Shen W, Punyanitya M, Chen J, et al. Visceral adipose tissue: relationships between single slice areas at different locations and obesity-related health risks. Int J Obes (2005). 2007;31:763–9.
Fogelman DR, Holmes H, Mohammed K, et al. Does IGFR1 inhibition result in increased muscle mass loss in patients undergoing treatment for pancreatic cancer? J Cachexia Sarcopenia Muscle. 2014;5:307–13.
Barret M, Antoun S, Dalban C, et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer. 2014;66:583–9.
Wakabayashi H, Sakuma K. Rehabilitation nutrition for sarcopenia with disability: a combination of both rehabilitation and nutrition care management. J Cachexia Sarcopenia Muscle. 2014.
Santa Mina D, Clarke H, Ritvo P, et al. Effect of totalbody prehabilitation on postoperative outcomes: a systematic review and meta-analysis. Physiotherapy. 2014;100:196–207.
de Campos-Ferraz PL, Andrade I, das Neves W, Hangai I, Alves CR, Lancha AH Jr. An overview of amines as nutritional supplements to counteract cancer cachexia. J Cachexia Sarcopenia Muscle. 2014;5:105–10.
Di Girolamo FG, Situlin R, Mazzucco S, Valentini R, Toigo G, Biolo G. Omega-3 fatty acids and protein metabolism: enhancement of anabolic interventions for sarcopenia. Curr Opin Clin Nutr Metab Care. 2014;17:145–50.
Morley JE, von Haehling S, Anker SD. Are we closer to having drugs to treat muscle wasting disease? J Cachexia Sarcopenia Muscle. 2014;5:83–7.
Hong DS, Hui D, Bruera E, et al. MABp1, a first-in-class true human antibody targeting interleukin-1alpha in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 2014;15:656–66.
Katz MH, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013;20:2787–95.
Acknowledgment
Supported by the Khalifa Bin Zayed Al Nahyan Foundation and by the Various Donor Pancreatic Research Fund at the University of Texas MD Anderson Cancer Center. ABC is also supported by a grant from the Foundation of Surgical Fellowships. RS is part of the Biostatistics Shared Resource supported by the NIH/NCI under award number P30CA016672.
Disclosures
There are no relevant financial disclosures.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2014_4285_MOESM1_ESM.tif
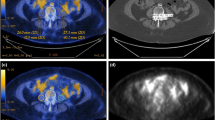
Figure S1. Anthropometric analysis. The cross sectional areas of the skeletal muscle (red), subcutaneous adipose tissue (light blue) and visceral adipose tissue (dark blue) compartments were computed using CT images obtained as part of routine care, and were standardized to the square of the height in meters. Supplementary material 1 (TIFF 831 kb)
Rights and permissions
About this article
Cite this article
Cooper, A.B., Slack, R., Fogelman, D. et al. Characterization of Anthropometric Changes that Occur During Neoadjuvant Therapy for Potentially Resectable Pancreatic Cancer. Ann Surg Oncol 22, 2416–2423 (2015). https://doi.org/10.1245/s10434-014-4285-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4285-2