Abstract
Introduction
Two randomized intraoperative radiation therapy trials for early-stage breast cancer were recently published. The ELIOT Trial used electrons (IOERT), and the TARGIT-A Trial Update used 50-kV X-rays (IORT). These studies were compared for similarities and differences. The results were analyzed and used to determine which patients might be suitable for single-dose treatment.
Method
The primary sources of data were the ELIOT Trial and TARGIT-A Trial, as well as a comprehensive analysis of the peer-reviewed literature of accelerated partial breast irradiation (APBI) using 50-kV X-rays or electrons. Studies published or presented prior to March 2014 were analyzed for efficacy, patient restrictions, complications, and outcome.
Results
With a median follow-up of 5.8 years, the 5-year recurrence rates for ELIOT versus external beam radiation therapy (EBRT) patients were 4.4 % and 0.4 %, respectively, p = .0001. A low-risk ELIOT group was identified with a 5-year recurrence rate of 1.5 %. With a median follow-up of 29 months, the 5-year recurrence rates for the TARGIT-A versus EBRT patients were 3.3 % and 1.3 %, respectively, p = .042.
Conclusion
With 5.8 years of median follow-up, IOERT appears to have a subset of low-risk women for whom IOERT is acceptable. With 29 months of median follow-up the results of IORT with 50-kV devices are promising, but longer follow-up data are required. At the current time, single-fraction IOERT or IORT patients should be treated under strict institutional protocols.
Similar content being viewed by others
When breast conserving surgery (BCS) is chosen, excision is commonly followed by 5 weeks of whole breast irradiation (WBI), with or without a boost to the tumor bed. Long radiation schedules are a burden for many women.1,2 This has stimulated an interest in accelerated partial breast irradiation (APBI) that can reduce overall treatment time without compromising oncological outcomes or cosmesis.3,4 Intraoperative radiation therapy (IORT) is an attractive APBI approach because it delivers the entire radiation treatment during surgery. Two randomized IORT-APBI trials, ELIOT using electrons and TARGIT-A using 50-kV X-rays, have studied whether IORT can produce results that are equivalent to standard treatment.5–7 In a series of 2 reports, we analyze these studies to determine whether IORT is ready for incorporation into standard practice and to determine what patient cohorts might be suitable for single-dose treatment.
Methods
The primary sources of data for these analyses were the ELIOT Trial and TARGIT-A Trials, as well as a comprehensive review of peer-reviewed literature of APBI studies using 50-kV X-rays or electrons, involving 50 or more patients with a minimum of 30 months median follow-up.5–7
Since the energy source for intraoperative radiation therapy and the technique used for delivery is different for ELIOT and TARGIT-A, each study is discussed in a separate report. The Results Section for each trial summarizes outcomes reported in the trial publications. The Discussion Section uses other studies as well as the trial publications to assess efficacy of the treatment and provide guidance on their use in non-trial environments. Intraoperative radiation therapy given with electrons (ELIOT) is referred to as IOERT. Intraoperative radiation therapy given with 50-kV x-rays (TARGIT A) is referred to as IORT.
Eliot Trial
Overview
The ELIOT Trial randomized 1,305 patients, 48 years or older, with tumors 2.5 cm or smaller to either a single dose of 21 Gy prescribed to the 90 % depth or to 50 Gy of external beam radiation therapy (EBRT) and a 10-Gy boost delivered over 6 weeks.5 With a median follow-up of 5.8 years, the 5-year recurrence rate was 4.4 % for ELIOT versus 0.4 % for the EBRT (p < .0001). The data are summarized in Table 1.
Technique 8
After tumor excision, the breast tissue was mobilized. The chest wall and underlying structures were protected with a lead/aluminum shield. The breast tissue to be irradiated was reapproximated over the shield. An appropriately sized collimator (4–8 cm) was inserted. Radiotherapy was performed using a linear accelerator; 21 Gy, to the 90 % isodose, was delivered to the tumor bed.
Complications
Compared with the conventional arm, ELIOT reported less skin damage (i.e., erythema, dryness, hyper-pigmentation, or itching), p = .0002, and no differences for fibrosis, retraction, pain or burning, but a higher incidence of radiologically determined fat necrosis, 5 %, versus 2 %, p = .04. In addition, ELIOT had less pulmonary toxicity than the EBRT as diagnosed by follow-up spiral CT (4 in the ELIOT arm and 38 in the EBRT arm). These differences in skin and pulmonary toxicity are not unexpected given the differences in IOERT versus EBRT breast irradiation techniques.
Local Recurrences
The 5-year ipsilateral breast tumor recurrence (IBTR) rates exceeded 10 % for patients with tumors >2 cm (10 of 83, 10.9 %), 4 or more positive nodes (4 of 31, 15.0 %), poorly differentiated tumors, i.e., grade 3 (15 of 129, 11.9 %), estrogen receptor negative tumors (8 of 63, 14.9 %), or triple negative disease (7 of 43, 18.9 %). Patients with a high proliferative index, i.e., Ki-67 > 20 %, trended to a high IBTR rate (22 of 244, 9.1 %) but did not reach the 10 % threshold. The 5-year IBTR was 11.3 % for the 199 women (30.6 %) with 1 or more of these risk factors vs 1.5 % for the 452 women (69.4 %) with none of these factors (ELIOT Low Risk). The per-protocol results were similar to the intent-to-treat analysis. The IBTR was 4.7 % versus 0.5 % for ELIOT versus EBRT; the 5-year IBTR was 11.8 % for the 178 women (30.4 %) with 1 or more risk factors versus 1.7 % for the 407 ELIOT Low Risk women (69.6 %).
Regional Failures
Greater regional failure with ELIOT (9 patients, 1.0 %) versus EBRT (2 patients, 0.3 %), p = .03, raised concern that fewer regional recurrences with EBRT might be partially due to lower axillary coverage by the tangential breast irradiation.
Patients with 4 or More Positive Nodes
A total of 69 patients with 4 or more positive nodes received additional EBRT of 50 Gy to the axilla. Those randomized to EBRT received axillary irradiation concurrently. Axillary irradiation was delayed 6–12 weeks for IOERT patients. Timing of adjuvant chemotherapy administration for these patients was not specified.
Elsewhere Recurrences
There were 14 (1.9 %) versus no (zero) ipsilateral “elsewhere” recurrences with ELIOT vs EBRT, p < .0001.
Contralateral Breast Cancer
There was a nonsignificant higher contralateral breast cancer rate in the EBRT group vs ELIOT (13 vs 8 patients).
Metastatic Breast Cancer
Metastases, other primary cancers, breast cancer death, and other deaths were similar in the 2 groups (Table 1).
Survival
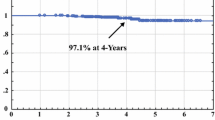
Overall survival at 5 years was identical, 96.8 % for the ELIOT group vs 96.9 % for the EBRT group. The 10-year survival remained similar (89.8 % for ELIOT and 92.0 % for EBRT patients).
Discussion
The ELIOT trial closed in December 2007. Analysis of the results began 5 years after accrual of the last patient. This is important since the time to local recurrence after radiation therapy combined with adjuvant treatment can be delayed.9,10 Overall, ELIOT patients had a higher 5-year recurrence rate than EBRT patients (4.4 % vs 0.4 %, p = .0001). However, ELIOT patients can be divided into low- and high-risk groups based on tumor size, receptor status, nodal positivity, and grade. ELIOT Low-Risk women (69.4 % of the ELIOT patients) had a 5-year IBTR rate of only 1.5 % compared with 11.3 % for the 30.6 % of ELIOT patients with 1 or more high-risk factors.
The American Society of Therapeutic Radiologists (ASTRO) has also published a set of criteria for selecting patients who are suitable for APBI.11 For the 23 % of the ELIOT patients who were ASTRO suitable for APBI, the IBTR was 1.5 % at 5 years and equivalent to the IBTR for the EBRT-suitable patients.12 The low recurrence for ASTRO-suitable women is consistent with a large series of patients treated with ELIOT off-protocol at the European Institute of Oncology (EIO).13,14 Table 2 shows local relapse rates for Out-Trial patients using the ASTRO and ESTRO APBI criteria and for favorable luminal A biology patients.11,15 The Out-Trial results show low 5-year recurrence rates for ASTRO suitable, ESTRO good, and Luminal A women, suggesting that favorable tumor biology might allow IOERT to obtain acceptable results in patients unsuitable for APBI by ASTRO or ESTRO guidelines.
The University of Verona reported only 1 recurrence at a mean follow-up of 46 months in 226 low-risk women treated with 21 Gy to D max (about 10 % lower dose than used in the ELIOT Trial).16 Updated results, first presented at the San Antonio Breast Cancer Symposium in 2012, showed only 4 recurrences (1.8 %) with a mean follow-up of 51 months.17 The median follow-up is now 5 years with no further recurrences.
In ELIOT patients, 14 of 35 (40 %) of the ipsilateral recurrences were “elsewhere” recurrences, raising the question of whether the applicator size might have been too small to adequately treat microscopic disease extending beyond the excised tumor. The ELIOT authors write: “The difficulty (with IOERT APBI) is not only to define patients at low risk of harboring microscopic disease beyond the tumor site, but also to define the proper coverage of the tumor bed.”5
This hints that larger applicators may have reduced recurrence rates, a concern confirmed by Leonardi et al.13 Noting the 4-cm median applicator size used in the ELIOT Out-Trial study and that the pattern of recurrences indicated neoplastic tumor foci outside the effective radiation field, she stated that they were planning to increase the field size used in ELIOT. With a 4-cm applicator, despite the IOERT surgical preparation bringing almost 2 cm of surrounding tissue under the applicator, only about 1.5 cm of surrounding tissue is irradiated to the prescription dose of 90 % because electron applicators have cold spots in the field periphery. Krechetov estimates that, depending on the energy, a 4-cm applicator covers at most only 55 % of the clinical treatment volume (“CTV”) to the 90 % prescription dose.18 To ensure uniform coverage of microscopic residual disease, the IOERT applicator should have a circumferential dimension at least 1.5 to 2 cm larger than the maximum tumor dimension. The applicator sizes used in the ELIOT Trial are not specified, but the current guidelines for ELIOT at the EIO (Table 3) indicating larger field sizes, as suggested by Leonardi, are now preferred.13
In Verona, where applicator size was selected to be approximately 2 cm circumferentially larger than the largest tumor dimension, median applicator size was 6 cm, 87 % were >5 cm, and 31 % were >6 cm, ensuring good coverage of the tumor bed.17
The lower dose used in Verona has lower toxicity and results in a higher percentage of the clinical treatment volume receiving the prescription dose. The higher dose used in the ELIOT study has acceptable toxicity at 5 years, but the higher ELIOT dose could impact longer-term cosmesis, especially when larger applicators are used.12
In the ELIOT Trial, 53 of 651 (8.1 %) of the IOERT patients had invasive lobular carcinoma (ILC).5 ILC is generally excluded in APBI trials. In the ELIOT study, ILC did not surface as an ELIOT high-risk factor, but 5 of 35 (14.3 %) of the total IOERT recurrences were from ILC or mixed IDC/ILC patients. Maluta, comparing his APBI patients with those in the ELIOT Out-Trial, found that ILC might be a risk factor (p = .04) in patient selection.16,19 The current ELIOT policy at the EIO permits ILC only after MRI assessment (Table 3).
Univariate analysis in the ELIOT Out-Trial showed that 3 or more positive nodes was a risk for recurrence, but not a factor in the multivariate analysis.19 However, annual rates of recurrence with 3 or more positive nodes compared unfavorably with patients with 0 or 1–2 positive nodes: for true recurrences (1.57 vs 0.69 % or 0.70 %), ipsilateral breast elsewhere recurrences (1.35 % vs 0.29 % or 0.69 %), and annual rates of breast cancer deaths (2.97 vs 0.52 % or 0.70 %).19 The University of Verona included 50 patients (22.1 %) that had either a positive SNB at the time of surgery or final pathology who also underwent complete axillary lymph node dissection during the initial surgery or at a second operation.16 A total of 38 patients had 1 positive lymph node and 12 had 2. All received IOERT. None of the recurrences at 5 years (Table 4) had a positive sentinel node. In the ELIOT Trial patients were stratified as N0, 1–3 positive nodes, or 4 or more positive nodes.5 The presence of 4 or more positive nodes disqualified a patient as ELIOT Low Risk. Patients with 1–3 positive nodes had a 5-year recurrence rate of 5.3 %, and 10 of 35 (28 %) of the recurrences came from this group. It would have been instructive to see if patients with only 1 or 2 positive nodes had a lower recurrence rate as the ELIOT Out-Trial suggests.19 It would also allow treatment decisions to be made per the American Society of Breast Surgeons guidelines based on the ACOSOG Z0011 treatment of patients with 1 or 2 positive nodes.20,21
Some IOERT APBI centers perform definitive node assessment in a separate out-patient surgery prior to tumor removal, and only patients with pN0 receive IOERT APBI. While this resolves nodal status, it subjects all patients to a second surgical procedure that 70–75 % will not require.
Margins are not discussed, but we know from previous ELIOT presentations that the positive margin rate was very low: only 3 (0.5 %) in the ELIOT arm and 9 (1.4 %) in the EBRT arm.22 Other centers treating with IOERT APBI that find positive margins either re-excise or ignore them, with no apparent impact on recurrence reported to date. In the Maluta study, none of the 16 patients (7.1 %) with positive margins or the 17 patients (7.5 %) with close margins have recurred.16 Jobsen has demonstrated that margin positivity in older women does not seem to impact recurrence.23 More data are needed to determine the impact of positive margins on recurrence.
APBI requires proper patient selection and proper technical implementation. The ELIOT Trial and other APBI IOERT published studies show a probable subset of women who can safely benefit from this 1-day treatment. These are the ELIOT Low Risk, ASTRO suitable, ESTRO good, or Luminal A patients. The current guidelines for ELIOT at the EIO are shown in Table 3.
The ELIOT authors suggest that preoperative criteria (e.g., tumor size, age, and pathological and biological examination of the biopsy specimen) can be used to identify suitable patients. A second option, they say, would be to treat all patients with IOERT, and after postsurgical categorization, give WBI to patients at high risk for recurrence. However, this is technically challenging, as the WBI should avoid overlap with the volume of tissue irradiated with IOERT. The better solution is to select APBI patients who are suitable for IOERT and who can then be expected to have low 5-year recurrence rates with no adverse impact on overall survival.
ELIOT Conclusions
The ELIOT trial has contributed to our understanding of whether a single-dose treatment using electrons may be possible. The Trial included some high-risk patients that today would not be considered a good choice for APBI. It appears, however, that IOERT APBI may have a subset of low-risk women (ASTRO suitable, ELIOT Low Risk, Luminal A) for whom IOERT could be effective, with a recurrence rate in the 2 % range at 5 years. In spite of a 5.8-year median follow-up, the ELIOT data are still early and single-fraction IOERT patients should be treated under strict institutional protocols. When long-term results are available, it is likely there will be a higher overall recurrence rate for IOERT when compared with EBRT, but we should be able to select subgroups of favorable patients where this difference is small and acceptable. How much additional risk of local recurrence is acceptable will vary with patients and the situation in which they find themselves. Overall, the results of the ELIOT Trial are reasonably mature and encouraging.
References
Athas W, Adams-Cameron M, Hunt WC, Amir-Fazli A, Key C. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst. 2000;92:269–71.
Ballard-Barbash R, Potosky A, Harlan L, Nayfield S, Kessler L. Factors associated with surgical and radiation therapy for early stage breast cancer in older women. J Natl Cancer Inst. 1996;88:716–26.
Polgar C, Fodor J, Tibor M, Sulyok Z, Hasler M. Breast-conserving therapy with partial or whole breast radiation: Ten-year results of the Budapest randomized trial. Radiother Oncol. 2013;108:197–202.
Shah C, Badiyan S, Wilkinson J, Vicini F, Beitsch P, Keisch M, et al. Treatment efficacy with accelerated partial breast irradiation (APBI): final analysis of the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial. Ann Surg Oncol. 2013;20:3279–85.
Veronesi U, Orecchia R, Maisonneuve P, Viale G, Rotmensz N, Sangalli C, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomized controlled equivalence trial. Lancet Oncol. 2013;14:1269–77.
Vaidya JS, Joseph DJ, Tobias JS, Bulsara M, Wenz F, Saunders C, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376:91–102.
Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomized trial. Lancet. 2013;383:603–13.
Veronesi U, Gatti G, Luini A, Intra M, Orecchia R, Borgen P, et al. Intraoperative radiation therapy for breast cancer: technical notes. Breast J. 2003;9:106–12.
Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–16.
Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31:2382–7.
Smith BD, Arthur DW, Buchholz TA, Haffty BG, Hahn CA, Hardenbergh PH, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). J Am Coll Surg. 2009;209:269–77.
Orecchia R, Leonardi M, Maisonneuve P, Morra A, Lazzari R, Cattani F, et al. Intraoperative Radiotherapy with electrons (ELIOT) for early breast cancer: the European Institute of Oncology experience. Transl Cancer Res. 2014;3:59–64.
Leonardi MC, Maisonneuve P, Mastropasqua MG, Morra A, Lazzari R, Dell’Acqua V, et al. Accelerated partial breast irradiation with intraoperative electrons: Using GEC-ESTRO recommendations as guidance for patient selection. Radiother Oncol. 2013;106:21–7.
Leonardi MC, Maisonneuve P, Mastropasqua MG, Morra A, Lazzari R, Rotmensz N, et al. How do the ASTRO consensus statement guidelines for the application of accelerated partial breast irradiation fit intraoperative radiotherapy? A retrospective analysis of patients treated at the European Institute of Oncology. Int J Radiat Oncol Biol Phys. 2012;83:806–13.
Polgár C, Van Limbergen E, Pötter R, Kovács G, Polo A, Lyczek J, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence. Radiother Oncol. 2010;94:264–73.
Maluta S, Dall’Oglio S, Marciai N, Gabbani M, Franchini Z, Pietrarota P, et al. Accelerated partial breast irradiation using only intraoperative electron radiation therapy in early stage breast cancer. Int J Radiat Oncol Biol Phys. 2012;84:e145–52.
Maluta S, Dall’Oglio S, Goer D, Marcial N. Intraoperative electron radiotherapy (IOERT) as an alternative to standard whole breast irradiation: only for low-risk subgroups? Breast Care (Basel). 2014;9:102–6.
Krechetov S, Goer D. Effective Treatment Volume of the Small Size IORT Applicators. Paper presented at: American Association of Physicists in Medicine. July 20–24, 2014; Austin, TX.
Veronesi U, Orecchia R, Luini A, Galimberti V, Zurrida S, Intra M, et al. Intraoperative radiotherapy during breast conserving surgery: a study on 1,822 cases treated with electrons. Breast Cancer Res Treat. 2010;124:141–51.
Surgeons TASoB. Position Statement on Management of the Axilla in Patients with Invasive Breast Cancer. The American Society of Breast Surgeons, 2011. https://www.breastsurgeons.org/statements/PDF_Statements/Axillary_Management.pdf.
Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–32.
Veronesi U. The Paradigm of Early Breast Cancer: Opportunities for IORT. Paper presented at: ISIORT 2008, International Society of Intraoperative Radiation Therapy, June 11, 2014, Madrid, Spain.
Jobsen J, van der Palen J, Ong F, Meerwaldt J. The value of a positive margin for invasive carcinoma in breast-conservative treatment in relation to local recurrence is limited to young women only. Int J Radiat Oncol Biol Phys. 2003;57:724–31.
Goer D, Silverstein MJ. The emerging role of intraoperative radiation therapy (IORT) in breast cancer. In: Riker A, Krueger B (eds) Breast disease: comprehensive management. New York, Heidelberg, London: Springer Science; 2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Silverstein, M.J., Fastner, G., Maluta, S. et al. Intraoperative Radiation Therapy: A Critical Analysis of the ELIOT and TARGIT Trials. Part 1—ELIOT. Ann Surg Oncol 21, 3787–3792 (2014). https://doi.org/10.1245/s10434-014-3998-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-3998-6