Abstract
Background
Despite recent advances in earlier detection and improvements in chemotherapy, the 5-year survival rate of patients with metastatic colorectal carcinoma remains poor. Immunotherapy is a potentially effective therapeutic approach to the treatment of colorectal carcinoma. Preclinical studies have supported the antitumor activity of immunization with a granulocyte–macrophage colony-stimulating factor (GM-CSF) producing murine colon tumor cell vaccine.
Methods
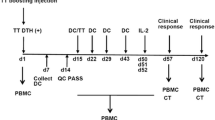
A novel colorectal cancer vaccine composed of irradiated, allogeneic human colon cancer cells and GM-CSF-producing bystander cells was developed and tested in combination with a single intravenous low dose of cyclophosphamide in a phase 1 study of patients with metastatic colorectal cancer.
Results
A total of nine patients were enrolled onto and treated in this study. Six patients had a history of colorectal adenocarcinoma hepatic metastases and underwent curative metastasectomy, while three other patients had unresectable stage IV disease. This study demonstrates the safety and feasibility of this vaccine administered in patients with metastatic colorectal cancer. At last follow-up, the six patients who underwent curative metastasectomy survived longer than 36 months, and four of these six patients were without disease recurrence. Immunologic correlate results suggest that the GM-CSF-producing colon cancer vaccine enhances the production of anti-MUC1 antibodies.
Conclusions
This vaccine is feasible and safe. Future investigation of the efficacy and antitumor immunity of this vaccine is warranted.


Similar content being viewed by others
References
Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomark Prev. 2010;19:1893–907.
Benson AB 3rd, Bekaii-Saab T, Chan E, et al. Localized colon cancer, version 3.2013: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. 2013;11:519–28.
Benson AB 3rd, Bekaii-Saab T, Chan E, et al. Metastatic colon cancer, version 3.2013: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. 2013;11:141–52.
Rothbarth J, van de Velde CJ. Treatment of liver metastases of colorectal cancer. Ann Oncol. 2005;16 Suppl 2:ii144–9.
Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22.
Mosolits S, Nilsson B, Mellstedt H. Towards therapeutic vaccines for colorectal carcinoma: a review of clinical trials. Expert Rev Vaccines. 2005;4:329–50.
Jain A, Slansky JE, Matey LC, et al. Synergistic effect of a granulocyte–macrophage colony-stimulating factor–transduced tumor vaccine and systemic interleukin-2 in the treatment of murine colorectal cancer hepatic metastases. Ann Surg Oncol. 2003;10:810–20.
Uyl-de Groot CA, Vermorken JB, Hanna MG Jr, et al. Immunotherapy with autologous tumor cell-BCG vaccine in patients with colon cancer: a prospective study of medical and economic benefits. Vaccine. 2005;23:2379–87.
Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer—science driving clinical progress. Nat Rev Cancer. 2005;5:459–67.
Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte–macrophage colony-stimulating factor–secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–56.
Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor–secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–63.
Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte–macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–35.
Thomas MC, Greten TF, Pardoll DM, Jaffee EM. Enhanced tumor protection by granulocyte–macrophage colony-stimulating factor expression at the site of an allogeneic vaccine. Hum Gene Ther. 1998;9:835–43.
Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte–macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–43.
Borrello I, Sotomayor EM, Cooke S, Levitsky HI. A universal granulocyte–macrophage colony-stimulating factor–producing bystander cell line for use in the formulation of autologous tumor cell-based vaccines. Hum Gene Ther. 1999;10:1983–91.
Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81.
Banham AH, Powrie FM, Suri-Payer E. FOXP3 + regulatory T cells: current controversies and future perspectives. Eur J Immunol. 2006;36:2832–6.
Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology. 2013;144:1230–40.
Ercolini AM, Ladle BH, Manning EA, et al. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–602.
Leao IC, Ganesan P, Armstrong TD, Jaffee EM. Effective depletion of regulatory T cells allows the recruitment of mesothelin-specific CD8 t cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinoma. Clin Transl Sci. 2008;1:228–239.
Emens LA, Asquith JM, Leatherman JM, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte–macrophage colony-stimulating factor–secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–8.
Shawler DL, Bartholomew RM, Garrett MA, et al. Antigenic and immunologic characterization of an allogeneic colon carcinoma vaccine. Clin Exp Immunol. 2002;129:99–106.
Yamamoto Y, Hirakawa E, Mori S, et al. Cleavage of carcinoembryonic antigen induces metastatic potential in colorectal carcinoma. Biochem Biophys Res Commun. 2005;333:223–9.
Takenaga K, Nakanishi H, Wada K, et al. Increased expression of S100A4, a metastasis-associated gene, in human colorectal adenocarcinomas. Clin Cancer Res. 1997;3:2309–16.
Simons JW, Jaffee EM, Weber CE, et al. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte–macrophage colony-stimulating factor gene transfer. Cancer Res. 1997;57:1537–46.
Lubaroff DM. Prostate cancer vaccines in clinical trials. Expert Rev Vaccines. 2012;11:857–68.
Simons JW, Sacks N. Granulocyte–macrophage colony-stimulating factor–transduced allogeneic cancer cellular immunotherapy: the GVAX vaccine for prostate cancer. Urol Oncol. 2006;24:419–24.
Dittmann J, Keller-Matschke K, Weinschenk T, et al. CD8 + T-cell response against MUC1-derived peptides in gastrointestinal cancer survivors. Cancer Immunol Immunother. 2005;54:750–8.
Mukherjee P, Pathangey LB, Bradley JB, et al. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine. 2007;25:1607–18.
Schimanski CC, Mohler M, Schon M, et al. LICC: L-BLP25 in patients with colorectal carcinoma after curative resection of hepatic metastases: a randomized, placebo-controlled, multicenter, multinational, double-blinded phase II trial. BMC Cancer. 2012;12:144.
Morse MA, Niedzwiecki D, Marshall JL, et al. A randomized phase II study of immunization with dendritic cells modified with poxvectors encoding CEA and MUC1 compared with the same poxvectors plus GM-CSF for resected metastatic colorectal cancer. Ann Surg. 2013;258:879–86.
Acknowledgment
This work was supported by NIH K23 CA 104160-05 and NIH R01 CA112160 to R. S. L. Z. was supported by NIH K23 CA148964, a Johns Hopkins University School of Medicine Clinician Scientist Award, Lefkofsky Family Foundation, the NCI SPORE in Gastrointestinal Cancers P50 CA062924, and the Zhang Family Gift Fund/Susan Cohan Colon Cancer Foundation. E. M. J. was supported by the NCI SPORE in Gastrointestinal Cancers P50 CA062924.
Disclosure
Under a licensing agreement between Aduro Biotech and the Johns Hopkins University (JHU), the university and investigators are entitled to milestone payments and royalty on sales of the GM-CSF-secreting colon tumor vaccine product described herein.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lei Zheng and Barish H. Edil contributed equally to this article, and both should be considered first author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, L., Edil, B.H., Soares, K.C. et al. A Safety and Feasibility Study of an Allogeneic Colon Cancer Cell Vaccine Administered with a Granulocyte–Macrophage Colony Stimulating Factor–Producing Bystander Cell Line in Patients with Metastatic Colorectal Cancer. Ann Surg Oncol 21, 3931–3937 (2014). https://doi.org/10.1245/s10434-014-3844-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-3844-x