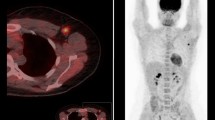
Abstract
Background
If all initially node-positive patients undergo axillary lymph node dissection (ALND) after neoadjuvant chemotherapy (NAC), overtreatment may occur in patients with complete response. Positron emission tomography–computed tomography (PET/CT) during NAC may predict axillary response and select patients appropriate for less invasive treatment after NAC. We evaluated the value of sequential 18F fluorodeoxyglucose (FDG) PET/CTs during NAC for axillary response monitoring in stage II–III breast cancer.
Methods
A total of 219 PET/CTs were performed in 80 patients with cytology-proven, node-positive disease at baseline (PET/CT1, n = 80) and twice during NAC (PET/CT2 n = 62, PET/CT3, n = 77). The relative changes in maximum standardized uptake value (SUVmax) of axillary nodes were examined for their ability to assess pathological response. All patients underwent ALND after chemotherapy, and complete axillary response (pCR), defined as absence of isolated tumor cells and of micro- and macrometastases, served as the reference standard.
Results
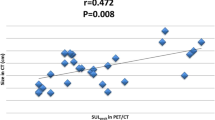
A total of 32 (40 %) patients experienced axillary pCR. The relative decrease in SUVmax was significantly higher in patients with pCR than in those without, both on PET/CT2 (p < 0.001) and PET/CT3 (p = 0.025). The area under the receiver operating characteristic curve values for PET/CT2 and PET/CT3 were 0.80 (95 % confidence interval 0.68–0.92) and 0.65 (95 % confidence interval 0.52–0.79), respectively. A relative decrease of ≥60 % on PET/CT2 had an excellent specificity (35 of 37, 95 %), a high positive predictive value (12 of 14, 86 %), and a sensitivity of 48 %—that is, it accurately identified histologic pCR in 12 of 25 patients with disease that responded to therapy.
Conclusions
18F-FDG PET/CT early during NAC is useful for axillary response monitoring in cytology-proven node-positive breast cancer because it identifies pathological response, thus permitting ALND to be spared.


Similar content being viewed by others
References
Kaufmann M, von Minckwitz G, Mamounas EP, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19:1508–16.
Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg. 2007;94:1189–200.
Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project protocols B-18 and B-27. J Clin Oncol. 2008;26:778–85.
von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–804.
von Minckwitz G, Blohmer JU, Costa S, et al. Neoadjuvant chemotherapy adapted by interim response improves overall survival of primary breast cancer patients—results of the GeparTrio trial (abstract). Presented at: San Antonio Breast Cancer Symposium, San Antonio, TX, December 2011.
Schwarz-Dose J, Untch M, Tiling R, et al. Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J Clin Oncol. 2009;27:535–41.
Loo CE, Straver ME, Rodenhuis S, et al. Magnetic resonance imaging response monitoring of breast cancer during neoadjuvant chemotherapy: relevance of breast cancer subtype. J Clin Oncol. 2011;29:660–6.
Kaufmann M, Karn T, Ruckhäberle E. Controversies concerning the use of neoadjuvant systemic therapy for primary breast cancer. World J Surg. 2012;36:1480–5.
Straver ME, Rutgers EJ, Russell NS, et al. Towards rational axillary treatment in relation to neoadjuvant therapy in breast cancer. Eur J Cancer. 2009;45:2284–92.
Gimbergues P, Abrial C, Durando X, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy is accurate in breast cancer patients with a clinically negative axillary nodal status at presentation. Ann Surg Oncol. 2008;15:1316–21.
Pecha V, Kolarik D, Kozevnikova R, et al. Sentinel lymph node biopsy in breast cancer patients treated with neoadjuvant chemotherapy. Cancer. 2011;117:4606–16.
Pritchard KI, Julian JA, Holloway CM, et al. Prospective study of 2-[18F]fluorodeoxyglucose positron emission tomography in the assessment of regional nodal spread of disease in patients with breast cancer: an Ontario clinical oncology group study. J Clin Oncol. 2012;30:1274–9.
Koolen BB, Valdés Olmos RA, Elkhuizen PH, et al. Locoregional lymph node involvement on 18F-FDG PET/CT in breast cancer patients scheduled for neoadjuvant chemotherapy. Breast Cancer Res Treat. 2012;135:231–40.
Straver ME, Loo CE, Alderliesten T, Rutgers EJ, Vrancken Peeters MJ. Marking the axilla with radioactive iodine seeds (MARI procedure) may reduce the need for axillary dissection after neoadjuvant chemotherapy for breast cancer. Br J Surg. 2010;97:1226–31.
Jung SY, Kim SK, Nam BH, et al. Prognostic Impact of [18F] FDG-PET in operable breast cancer treated with neoadjuvant chemotherapy. Ann Surg Oncol. 2010;17:247–53.
Rousseau C, Devillers A, Campone M, et al. FDG PET evaluation of early axillary lymph node response to neoadjuvant chemotherapy in stage II and III breast cancer patients. Eur J Nucl Med Mol Imaging. 2011;38:1029–36.
Koolen BB, Pengel KE, Wesseling J, et al. FDG PET/CT during neoadjuvant chemotherapy may predict response in ER-positive/HER2-negative and triple negative, but not in HER2-positive breast cancer. Breast. 2013. doi:10.1016/j.breast.2012.12.020.
Loo CE, Teertstra HJ, Rodenhuis S, et al. Dynamic contrast-enhanced MRI for prediction of breast cancer response to neoadjuvant chemotherapy: initial results. AJR Am J Roentgenol. 2008;191:1331–8.
Vidal-Sicart S, Aukema TS, Vogel WV, Hoefnagel CA, Valdes Olmos RA. Added value of prone position technique for PET-TAC in breast cancer patients. Rev Esp Med Nucl. 2010;29:230–5.
Koolen BB, Vrancken Peeters MJ, Aukema TS, et al. 18F-FDG PET/CT as a staging procedure in primary stage II and III breast cancer: comparison with conventional imaging techniques. Breast Cancer Res Treat. 2012;131:117–26.
Boellaard R, Oyen WJ, Hoekstra CJ, et al. The Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi-centre trials. Eur J Nucl Med Mol Imaging. 2008;35:2320–33.
Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–84.
Gil-Rendo A, Martínez-Regueira F, Zornoza G, García-Velloso MJ, Beorlegui C, Rodriguez-Spiteri N. Association between [18F]fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br J Surg. 2009;96:166–70.
Groheux D, Giacchetti S, Moretti JL, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–35.
Humbert O, Berriolo-Riedinger A, Riedinger JM, et al. Changes in 18F-FDG tumor metabolism after a first course of neoadjuvant chemotherapy in breast cancer: influence of tumor subtypes. Ann Oncol. 2012;23:2572–7.
Quarles van Ufford HM, van Tinteren H, Stroobants SG, Riphagen II, Hoekstra OS. Added value of baseline 18F-FDG uptake in serial 18F-FDG PET for evaluation of response of solid extracerebral tumors to systemic cytotoxic neoadjuvant treatment: a meta-analysis. J Nucl Med. 2010;51:1507–16.
Prati R, Minami CA, Gornbein JA, Debruhl N, Chung D, Chang HR. Accuracy of clinical evaluation of locally advanced breast cancer in patients receiving neoadjuvant chemotherapy. Cancer. 2009;115:1194–202.
Rouzier R, Extra JM, Klijanienko J, et al. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol. 2002;20:1304–10.
Smith IC, Heys SD, Hutcheon AW, et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol. 2002;20:1456–66.
von Minckwitz G, Kümmel S, Vogel P, et al. Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase III randomized GeparTrio trial. J Natl Cancer Inst. 2008;100:542–51.
Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–8.
Acknowledgment
We gratefully thank Marjo Holtkamp, Margaret Schot, and Jacqueline van Zyll de Jong for their active participation and guidance of patients throughout this study. This study was performed within the framework of CTMM, the Center for Translational and Molecular Medicine (http://www.ctmm.nl), project Breast CARE (grant 03O-104).
Disclosure
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2013_2902_MOESM1_ESM.tif
Supplementary material 1 (TIFF 4490 kb). Flow chart of eligibility, exclusion, inclusion, and number of PET/CT scans during neoadjuvant chemotherapy.Abbreviation: NAC, neoadjuvant chemotherapy; FNA, fine needle aspiration
10434_2013_2902_MOESM2_ESM.tif
Supplementary material 2 (TIFF 2426 kb). Fused PET/CT images of a tumor-positive lymph node in the right axilla before (A), after one course (B), and after three courses (C) of neoadjuvant chemotherapy. SUVmax was 12.0 on PET/CT1, 7.4 on PET/CT2, and 4.4 on PET/CT3. In the axillary lymph node dissection after six cycles of chemotherapy (twelve weeks) five macrometastases were found
Rights and permissions
About this article
Cite this article
Koolen, B.B., Valdés Olmos, R.A., Wesseling, J. et al. Early Assessment of Axillary Response with 18F-FDG PET/CT during Neoadjuvant Chemotherapy in Stage II–III Breast Cancer: Implications for Surgical Management of the Axilla. Ann Surg Oncol 20, 2227–2235 (2013). https://doi.org/10.1245/s10434-013-2902-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-013-2902-0