Abstract
Purpose
The prognostic implication of breast cancer with nodal micrometastases measuring >0.2 mm but ≤2 mm (pNmic) is unclear. This study evaluates survival in pNmic relative to node-negative (N0) and macroscopic node-positive (pNmac) disease in a large population-based series.
Methods
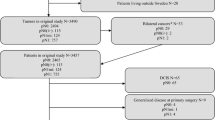
Subjects were 9,637 women diagnosed between 1989 and 1999, referred to the British Columbia Cancer Agency with pT1–2, node-negative and node-positive, M0 breast cancer. Kaplan–Meier breast-cancer-specific survival (BCSS) and overall survival (OS) were compared between patients with pN0 (n = 7,988), pNmic (n = 491), and pNmac disease (n = 1,158), according to the number of positive nodes and the lymph node ratio (LNR) of positive to excised nodes. Cox regression and recursive partitioning analyses were performed to identify significant factors associated with survival.
Results
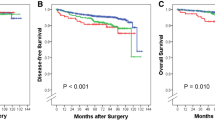
Median follow-up was 8.2 years. Patients with pNmic disease had significantly poorer outcomes compared with pN0 cancers, with progressively lower BCSS and OS with increasing number of positive nodes and with LNR > 0.25. On multivariable analysis, histologic subtype, T stage, number of positive nodes, LNR, grade, lymphovascular invasion, estrogen receptor status, and systemic therapy use were factors significantly associated with BCSS and OS. Recursive partitioning trees for BCSS and OS both selected the pN/LNR variable at the first split, indicating that this variable provided the strongest prognostic separation.
Conclusion
Patients with nodal micrometastases are a heterogeneous population with varying breast cancer mortality risks. The number of positive nodes and the LNR should be considered in conjunction with tumor factors in risk estimates and treatment decisions for patients with nodal micrometastatic breast cancer.




Similar content being viewed by others
References
Hawes D, Neville AM, Cote RJ. Detection of occult metastasis in patients with breast cancer. Semin Surg Oncol. 2001;20:312–8.
Cronin-Fenton DP, Ries LA, Clegg LX, Edwards BK. Rising incidence rates of breast carcinoma with micrometastatic lymph node involvement. J Natl Cancer Inst. 2007;99:1044–9.
Grabau D. Breast cancer patients with micrometastases only: is a basis provided for tailored treatment? Surg Oncol. 2008;17:211–7.
Wada N, Imoto S. Clinical evidence of breast cancer micrometastasis in the era of sentinel node biopsy. Int J Clin Oncol. 2008;13:24–32.
American Joint Committee on Cancer. Breast. In: AJCC cancer staging manual, 5th edition. Lippincott-Raven, Philadelphia; 1997.
Huvos AG, Hutter RV, Berg JW. Significance of axillary macrometastases and micrometastases in mammary cancer. Ann Surg. 1971;173:44–6.
Attiyeh FF, Jensen M, Huvos AG, Fracchia A. Axillary micrometastasis and macrometastasis in carcinoma of the breast. Surg Gynecol Obstet. 1977;144:839–42.
Fisher ER, Palekar A, Rockette H, Redmond C, Fisher B. Pathologic findings from the National Surgical Adjuvant Breast Project (Protocol No. 4). V. Significance of axillary nodal micro- and macrometastases. Cancer. 1978;42:2032–8.
Rosen PP, Saigo PE, Braun DW, Weathers E, Fracchia AA, Kinne DW. Axillary micro- and macrometastases in breast cancer: prognostic significance of tumor size. Ann Surg. 1981;194:585–91.
International (Ludwig) Breast Cancer Study Group. Prognostic importance of occult axillary lymph node micrometastases from breast cancers. Lancet. 1990;335:1565–8.
Cummings MC, Walsh MD, Hohn BG, et al. Occult axillary lymph node metastases in breast cancer do matter: results of 10-year survival analysis. Am J Surg Pathol. 2002;26:1260–95.
Chen SL, Hoehne FM, Giulano AE. The prognostic significance of micrometastases in breast cancer: a SEER population-based analysis. Ann Surg Oncol. 2007;14:3378–84.
Colleoni M, Rotmensz N, Peruzzotti G, et al. Size of breast cancer metastases in axillary lymph nodes: clinical relevance of minimal lymph node involvement. J Clin Oncol. 2005;23:1379–89.
Truong PT, Vinh-Hung V, Cserni G, Woodward WA, Tai P, Vlastos G. The number of positive nodes and the ratio of positive to excised nodes are significant predictors of survival in women with micrometastatic node-positive breast cancer. Eur J Cancer 2008;44:1670–7.
de Boer M, van Deurzen CHM, van Dijck J, et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med. 2009;361:653–63.
Tan LK, Giri D, Hummer AJ, et al. Occult axillary node metastases in breast cancer are prognostically significant: results in 368 node-negative patients with 20-year follow-up. J Clin Oncol. 2008;26:1803–9.
Reed J, Rosman M, Verbanac KM, Mannie A, Cheng Z, Tafra L. Prognostic implications of isolated tumor cells and micrometastases in sentinel nodes of patients with invasive breast cancer: 10-year analysis of patients enrolled in the prospective East Carolina University/Anne Arundel Medical Center Sentinel Node Multicenter Study. J Am Coll Surg. 2009;208:333–40.
Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging handbook. TNM classification of malignant tumors, 6th edition. Springer, New York; 2002.
Woodward WA, Vinh-Hung V, Ueno NT, et al. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol. 2006;24:2910–6.
Vinh-Hung V, Verschraegen C, Promish DI, et al. Ratios of involved nodes in early breast cancer. Breast Cancer Res. 2004;6:680–8.
van der Wal BC, Butzelaar RM, van der Meij S, Boermeester MA. Axillary lymph node ratio and total number of removed lymph nodes: predictors of survival in stage I and II breast cancer. Eur J Surg Oncol. 2002;28:481–9.
Yildirim E, Berberoglu U. Lymph node ratio is more valuable than level III involvement for prediction of outcome in node-positive breast carcinoma patients. World J Surg. 2007;31:276–89.
Vinh-Hung V, Verkooijen HM, Pioretto G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009;27:1062–8.
Kuru B. Prognostic significance of total number of nodes removed, negative nodes removed, and ratio of positive nodes to removed nodes in node positive breast carcinoma. Eur J Surg Oncol. 2006;32:1082–8.
Truong PT, Berthelet E, Lee J, Kader H, Olivotto IA. The prognostic significance of the percentage of positive/dissected axillary lymph nodes in breast cancer recurrence and survival in patients with one to three positive axillary lymph nodes. Cancer. 2005;103:2006–14.
Zhang H, Singer B. Statistics for biology and health: recursive partitioning in the health sciences. Springer, New York; 1999.
S-Plus 6 for Windows: Guide to Statistics, Volume 2. Insightful Corporation, Washington; 2001.
Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–20.
Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–53.
Cserni G, Bianchi S, Boecker W, et al. Improving the reproducibility of diagnosing micrometastases and isolated tumor cells. Cancer. 2005;103:358–67.
Turner RR, Weaver DL, Cserni G, et al. Nodal stage classification for breast carcinoma: improving interobserver reproducibility through standardized histologic criteria and image-based training. J Clin Oncol. 2008;26:258–63.
Gillanders WE, Mikhitarian K, Hebert R, et al. Molecular detection of micrometastatic breast cancer in histopathology-negative axillary lymph nodes correlated with traditional predictors of prognosis: an interim analysis of a prospective multi-institutional cohort study. Ann Surg. 2004;239:828–37.
Olivotto IA, Bajdik CD, Ravdin PM, et al. Population-based validation of the prognostic model Adjuvant! for early breast cancer. J Clin Oncol. 2005;23:2716–25.
Cserni G, Gregori D, Merletti F, et al. Non-sentinel node metastases associated with micrometastatic sentinel nodes in breast cancer: metaanalysis of 25 studies. Br J Surg. 2004;91:1245–52.
Viale G, Maiorano E, Mazzarol G, et al. Histologic detection and clinical implications of micrometastases in axillary sentinel lymph nodes for patients with breast carcinoma. Cancer. 2001;92:1378–84.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Truong, P.T., Lesperance, M., Li, K.H. et al. Micrometastatic Node-Positive Breast Cancer: Long-Term Outcomes and Identification of High-Risk Subsets in a Large Population-Based Series. Ann Surg Oncol 17, 2138–2146 (2010). https://doi.org/10.1245/s10434-010-0954-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-0954-y