Abstract
Background
Barrett esophagus develops in a scenario of chronic inflammation, linked to free radical formation and oxidative DNA damage. Eight-hydroxydeoxyguanosine, the main oxidative DNA adduct, is partially repaired by a glycosylase (OGG1) whose polymorphism is associated to a reduced repair capacity. Telomeres are particularly prone to oxidative damage, which leads to shortening and cell senescence, while elongation, by telomerase activity, is linked to cell immortalization and cancer. Limited data are available on this point with respect to Barrett esophagus. This study aimed to evaluate the link among 8-hydroxydeoxyguanosine, OGG1 polymorphism, telomerase activity, telomere length, and p53 mutation in Barrett progression.
Methods
Forty consecutive patients with short- and long-segment Barrett esophagus and 20 controls with gastroesophageal reflux disease without Barrett esophagus were recruited. Analysis of biopsy samples was undertaken to study 8-hydroxydeoxyguanosine levels, OGG1 polymorphism, telomerase activity, and telomere length. Serum samples were obtained for p53 mutation.
Results
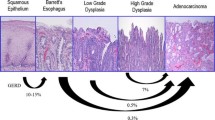
Controls had significantly lower levels of 8-hydroxydeoxyguanosine and telomerase activity, with normal telomere length and no p53 mutation. In short-segment Barrett esophagus, 8-hydroxydeoxyguanosine levels were higher and telomeres underwent significant shortening, with stimulation of telomerase activity but no p53 mutations. In long-segment Barrett esophagus, 8-hydroxydeoxyguanosine reached maximal levels, with telomere elongation, and 42 % of the patients showed p53 mutation.
Conclusions
In Barrett patients, with disease progression, oxidative DNA damage accumulates, causing telomere instability, telomerase activation, and, in a late phase, mutations in the p53 gene, thus abrogating its activity as the checkpoint of proliferation and apoptosis, and facilitating progression to cancer.



Similar content being viewed by others
References
Barrett NR. The lower esophagus lined by columnar epithelium. Surgery. 1957;41:881–94.
Spechler SJ. Clinical practice. Barrett’s esophagus. N Engl J Med. 2002;346:836–42.
Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: scientific review. JAMA. 2002;287:1972–81.
Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet. 2009;373:850–61.
Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084–91.
Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375–83.
Wiseman EF, Ang YS. Risk factors for neoplastic progression in Barrett’s esophagus. World J Gastroenterol. 2011;17:3672–83.
McManus DT, Olaru A, Meltzer SJ. Biomarkers of esophageal adenocarcinoma and Barrett’s esophagus. Cancer Res. 2004;64:1561–9.
Koppert LB, Wijnhoven BP, van Dekken H, Tilanus HW, Dinjens WN. The molecular biology of esophageal adenocarcinoma. J Surg Oncol. 2005;92:169–90.
Fang D, Das KM, Cao W, et al. Barrett’s esophagus: progression to adenocarcinoma and markers. Ann N Y Acad Sci. 2011;1232:210–29.
Olliver JR, Hardie LJ, Gong Y, et al. Risk factors, DNA damage, and disease progression in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2005;14:620–5.
Kadioglu E, Sardas S, Ergun M, Unal S, Karakaya AE. The role of oxidative DNA damage, DNA repair, GSTM1, SOD2 and OGG1 polymorphisms in individual susceptibility to Barrett’s esophagus. Toxicol Ind Health. 2010;26:67–79.
Zhang HY, Zhang Q, Zhang X, et al. Cancer-related inflammation and Barrett’s carcinogenesis: interleukin-6 and STAT3 mediate apoptotic resistance in transformed Barrett’s cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G454–60.
Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38:96–109.
Shinmura K, Yokota J. The OGG1 gene encodes a repair enzyme for oxidatively damaged DNA and is involved in human carcinogenesis. Antioxid Redox Signal. 2001;3:597–609.
Lee JS, Oh TY, Ahn BO, et al. Involvement of oxidative stress in experimentally induced reflux esophagitis and Barrett’s esophagus: clue for the chemoprevention of esophageal carcinoma by antioxidants. Mutat Res. 2001;480–1:189–200.
Rasanen JV, Sihvo EI, Ahotupa MO, Farkkila MA, Salo JA. The expression of 8-hydroxydeoxyguanosine in oesophageal tissues and tumours. Eur J Surg Oncol. 2007;33:1164–8.
Dvorak K, Payne CM, Chavarria M, et al. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett’s oesophagus. Gut. 2007;56:763–71.
De Ceglie A, Fisher DA, Filiberti R, Blanchi S, Conio M. Barrett’s esophagus, esophageal and esophagogastric junction adenocarcinomas: the role of diet. Clin Res Hepatol Gastroenterol. 2011;35:7–16.
Farinati F, Cardin R, Russo VM, et al. Differential effects of Helicobacter pylori eradication on oxidative DNA damage at the gastroesophageal junction and at the gastric antrum. Cancer Epidemiol Biomarkers Prev. 2004;13:1722–8.
Farinati F, Cardin R, Cassaro M, et al. Helicobacter pylori, inflammation, oxidative damage and gastric cancer: a morphological, biological and molecular pathway. Eur J Cancer Prev. 2008;17:195–200.
Rudolph KL, Hartmann D, Opitz OG. Telomere dysfunction and DNA damage checkpoints in diseases and cancer of the gastrointestinal tract. Gastroenterology. 2009;137:754–62.
Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9–18.
Maser RS, DePinho RA. Connecting chromosomes, crisis, and cancer. Science. 2002;297:565–9.
Shay JW, Wright WE. Role of telomeres and telomerase in cancer. Semin Cancer Biol. 2011;21:349–53.
Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology. 2006;131:1392–9.
Zaninotto G, Minnei F, Guirroli E, et al. The Veneto Region’s Barrett’s oesophagus registry: aims, methods, preliminary results. Dig Liver Dis. 2007;39:18–25.
Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–5.
Romilda C, Marika P, Alessandro S, et al. Oxidative DNA damage correlates with cell immortalization and mir-92 expression in hepatocellular carcinoma. BMC Cancer. 2012;12:177–85.
Kohno T, Shinmura K, Tosaka M, et al. Genetic polymorphisms and alternative splicing of the hOGG1 gene, that is involved in the repair of 8-hydroxyguanine in damaged DNA. Oncogene. 1998;16:3219–25.
Cawthon RM. Telomere measurement by quantitative PCR. Nucl Acids Res. 2002;30:e47.
Farinati F, Piciocchi M, Lavezzo E, Bortolami M, Cardin R. Oxidative stress and inducible nitric oxide synthase induction in carcinogenesis. Dig Dis. 2010;28:579–84.
Ide H, Kotera M. Human DNA glycosylases involved in the repair of oxidatively damaged DNA. Biol Pharm Bull. 2004;27:480–5.
Farinati F, Cardin R, Bortolami M, et al. Oxidative DNA damage in gastric cancer: CagA status and OGG1 gene polymorphism. Int J Cancer. 2008;123:51–5.
Ferguson LR, Laing WA. Chronic inflammation, mutation and human disease. Mutat Res. 2010;690:1–2.
Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391:499–510.
Kuchino Y, Mori F, Kasai H, et al. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987;327:77–9.
Fruehauf JP, Meyskens FL Jr. Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–94.
Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56.
Ock CY, Kim EH, Choi DJ, Lee HJ, Hahm KB, Chung MH. 8-Hydroxydeoxyguanosine: not mere biomarker for oxidative stress, but remedy for oxidative stress–implicated gastrointestinal diseases. World J Gastroenterol. 2012;18:302–8.
Savarino E, Zentilin P, Frazzoni M, et al. Characteristics of gastro-esophageal reflux episodes in Barrett’s esophagus, erosive esophagitis and healthy volunteers. Neurogastroenterol Motil. 2010;22:1061-e280.
Thrift AP, Kendall BJ, Pandeya N, Vaughan TL, Whiteman DC; Study of digestive health. A clinical risk prediction model for Barrett esophagus. Cancer Prev Res (Phila). 2012;5:1115–23.
Thanan R, Ma N, Iijima K, et al. Proton pump inhibitors suppress iNOS-dependent DNA damage in Barrett’s esophagus by increasing Mn-SOD expression. Biochem Biophys Res Commun. 2012;421:280–5.
Oikawa S, Kawanishi S. Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett. 1999;453:365–8.
Gertler R, Doll D, Maak M, Feith M, Rosenberg R. Telomere length and telomerase subunits as diagnostic and prognostic biomarkers in Barrett carcinoma. Cancer. 2008;112:2173–80.
Finley JC, Reid BJ, Odze RD, et al. Chromosomal instability in Barrett’s esophagus is related to telomere shortening. Cancer Epidemiol Biomarkers Prev. 2006;15:1451–7.
Vurusaner B, Poli G, Basaga H. Tumor suppressor genes and ROS: complex networks of interactions. Free Radic Biol Med. 2012;52:7–18.
Shields HM, Nardone G, Zhao J, et al. Barrett’s esophagus: prevalence and incidence of adenocarcinomas. Ann N Y Acad Sci. 2011;1232:230–47.
Soussi T. The humoral response to the tumor-suppressor gene-product p53 in human cancer: implications for diagnosis and therapy. Immunol Today. 1996;17:354–6.
Famulski W, Sulkowska M, Wincewicz A, et al. P53 correlates positively with VEGF in preoperative sera of colorectal cancer patients. Neoplasma. 2006;53:43–8.
Dobrzycka B, Terlikowski SJ, Kinalski M, Kowalczuk O, Niklinska W, Chyczewski L. Circulating free DNA and p53 antibodies in plasma of patients with ovarian epithelial cancers. Ann Oncol. 2011;22:1133–40.
Lubin R, Zalcman G, Bouchet L, et al. Serum p53 antibodies as early markers of lung cancer. Nat Med. 1995;1:701–2.
Broll R, Duchrow M, Oevermann E, et al. p53 autoantibodies in sera of patients with a colorectal cancer and their association to p53 protein concentration and p53 immunohistochemistry in tumor tissue. Int J Colorectal Dis. 2001;16:22–7.
Acknowledgment
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cardin, R., Piciocchi, M., Tieppo, C. et al. Oxidative DNA Damage in Barrett Mucosa: Correlation with Telomeric Dysfunction and p53 Mutation. Ann Surg Oncol 20 (Suppl 3), 583–589 (2013). https://doi.org/10.1245/s10434-013-3043-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-013-3043-1