Abstract
Background
The immediate early response gene X-1 (IEX-1) is a stress-inducible protein that is involved in the regulation of cell proliferation and apoptosis. The aim of this study was to evaluate the prognostic significance of IEX-1 expression in pancreatic cancer.
Methods
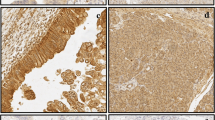
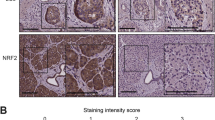
IEX-1 protein expression was examined on paraffin-embedded specimens from 78 patients with pancreatic ductal adenocarcinoma using immunohistochemistry. The relationships between the IEX-1 expression and other clinicopathological parameters and patient survival were evaluated. A similar analysis was conducted in a subgroup of 48 patients, who underwent a macroscopically curative resection with detailed information on the pathological findings.
Results
Among 78 pancreatic cancer patients, 41 patients (53%) were positive for IEX-1 staining. In a multivariate analysis, curative operation (P < .001), pathological stage I–III (P = .001), and positive IEX-1 expression (P = .002) were significantly favorable factors for survival. In a subgroup of 48 patients undergoing a macroscopically curative surgery, IEX-1 expression was positive in 28 patients (58%). A significant negative correlation was observed between the IEX-1 expression and serosal (P = .032) or arterial (P = .040) invasion of tumors. A multivariate analysis demonstrated limited local invasion (pT1-3, P = .021), negative lymph node involvement (pN0, P < .001), and positive IEX-1 expression (P = .004) to be significantly favorable factors for survival.
Conclusions
The positive IEX-1 expression in tumor tissues may be associated with a better prognosis in pancreatic cancer. An immunohistochemical assessment of IEX-1 expression may therefore be helpful for predicting patient prognosis in this disease.




Similar content being viewed by others
REFERENCES
Lockhart AC, Rothenberg ML, Berlin JD. Treatment for pancreatic cancer: current therapy and continued progress. Gastroenterology 2005;128:1642–54
Alexakis N, Halloran C, Raraty M, Ghaneh P, Sutton R, Neoptolemos JP. Current standards of surgery for pancreatic cancer. Br J Surg 2004;91:1410–27
Mimeault M, Brand RE, Sasson AA, Batra SK. Recent advances on the molecular mechanisms involved in pancreatic cancer progression and therapies. Pancreas 2005;31:301–16
Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 2006;20:1218–49
Jimeno A, Hidalgo M. Molecular biomarkers: their increasing role in the diagnosis, characterization, and therapy guidance in pancreatic cancer. Mol Cancer Ther 2006;5:787–96
Charles CH, Yoon JK, Simske JS, Lau LF. Genomic structure, cDNA sequence, and expression of gly96, a growth factor-inducible immediate-early gene encoding a short-lived glycosylated protein. Oncogene 1993;8:797–801
Kondratyev AD, Chung KN, Jung MO. Identification and characterization of a radiation-inducible glycosylated human early-response gene. Cancer Res 1996;56:1498–502
Schafer H, Trauzold A, Siegel EG, Folsch UR, Schmidt WE. PRG1: a novel early-response gene transcriptionally induced by pituitary adenylate cyclase activating polypeptide in a pancreatic carcinoma cell line. Cancer Res 1996;56:2641–8
Pietzsch A, Buchler C, Aslanidis C, Schmitz G. Identification and characterization of a novel monocyte/macrophage differentiation-dependent gene that is responsive to lipopolysaccharide, ceramide, and lysophosphatidylcholine. Biochem Biophys Res Commun 1997;235:4–9
Wu MX. Roles of the stress-induced gene IEX-1 in regulation of cell death and oncogenesis. Apoptosis 2003;8:11–8
Zhang Y, Schlossman SF, Edwards RA, Ou CN, Gu J, Wu MX. Impaired apoptosis, extended duration of immune responses, and a lupus-like autoimmune disease in IEX-1-transgenic mice. Proc Natl Acad Sci U S A 2002;99:878–83
Zhang Y, Finegold MJ, Porteu F, Kanteti P, Wu MX. Development of T-cell lymphomas in Emu-IEX-1 mice. Oncogene 2003;22:6845–51
Schilling D, Pittelkow MR, Kumar R. IEX-1, an immediate early gene, increases the rate of apoptosis in keratinocytes. Oncogene 2001;20:7992–7
Grobe O, Arlt A, Ungefroren H, et al. Functional disruption of IEX-1 expression by concatemeric hammerhead ribozymes alters growth properties of 293 cells. FEBS Lett 2001;494:196–200
Arlt A, Grobe O, Sieke A, et al. Expression of the NF-kappa B target gene IEX-1 (p22/PRG1) does not prevent cell death but instead triggers apoptosis in Hela cells. Oncogene 2001;20:69–76
Arlt A, Kruse ML, Breitenbroich M, et al. The early response gene IEX-1 attenuates NF-kappaB activation in 293 cells, a possible counter-regulatory process leading to enhanced cell death. Oncogene 2003;22:3343–51
Osawa Y, Nagaki M, Banno Y, et al. Expression of the NF-kappa B target gene X-ray-inducible immediate early response factor-1 short enhances TNF-alpha-induced hepatocyte apoptosis by inhibiting Akt activation. J Immunol 2003;170:4053–60
Nambiar PR, Nakanishi M, Gupta R, et al. Genetic signatures of high- and low-risk aberrant crypt foci in a mouse model of sporadic colon cancer. Cancer Res 2004;64:6394–401
Dilley WG, Kalyanaraman S, Verma S, Cobb JP, Laramie JM, Lairmore TC. Global gene expression in neuroendocrine tumors from patients with the MEN1 syndrome. Mol Cancer 2005;4:9
Hu Y, Sun H, Drake J, et al. From mice to humans: identification of commonly deregulated genes in mammary cancer via comparative SAGE studies. Cancer Res 2004;64:7748–55
Yang C, Trent S, Ionescu-Tiba V, et al. Identification of cyclin D1- and estrogen-regulated genes contributing to breast carcinogenesis and progression. Cancer Res 2006;66:11649–58
Japan Pancreas Society. Classification of Pancreatic Carcinoma, 2nd English ed. Tokyo: Kanehara; 2003
Sasada T, Takedatsu H, Azuma K, et al. Immediate early response gene X-1, a stress-inducible antiapoptotic gene, encodes cytotoxic T-lymphocyte (CTL) epitopes capable of inducing human leukocyte antigen-A33-restricted and tumor-reactive CTLs in gastric cancer patients. Cancer Res 2004;64:2882–8
Schafer H, Lettau P, Trauzold A, Banasch M, Schmidt WE. Human PACAP response gene 1 (p22/PRG1): proliferation-associated expression in pancreatic carcinoma cells. Pancreas 1999;18:378–84
Garcia J, Ye Y, Arranz V, Letourneux C, Pezeron G, Porteu F. IEX-1: a new ERK substrate involved in both ERK survival activity and ERK activation. EMBO J 2002;21:5151–63
Gonzalez S, Perez-Perez MM, Hernando E, Serrano M, Cordon-Cardo C. p73beta-Mediated apoptosis requires p57kip2 induction and IEX-1 inhibition. Cancer Res 2005;65:2186–92
Kumar R, Lutz W, Frank E, Im HJ. Immediate early gene X-1 interacts with proteins that modulate apoptosis. Biochem Biophys Res Commun 2004;323:1293–8
Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol 2002;3:999–1005
Matsueda S, Takedatsu H, Sasada T, et al. New peptide vaccine candidates for epithelial cancer patients with HLA-A3 supertype alleles. J Immunother 2007;30:274–81
Maleno I, Cabrera CM, Cabrera T, et al. Distribution of HLA class I altered phenotypes in colorectal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Immunogenetics 2004;56:244–53
Ryschich E, Cebotari O, Fabian OV, et al. Loss of heterozygosity in the HLA class I region in human pancreatic cancer. Tissue Antigens 2004;64:696–702
Kisseljov F, Semionova L, Samoylova E, et al. Instability of chromosome 6 microsatellite repeats in human cervical tumors carrying papillomavirus sequences. Int J Cancer 1996;69:484–7
Virmani AK, Fong KM, Kodagoda D, et al. Allelotyping demonstrates common and distinct patterns of chromosomal loss in human lung cancer types. Genes Chromosomes Cancer 1998;21:308–19
Chatterjee A, Pulido HA, Koul S, et al. Mapping the sites of putative tumor suppressor genes at 6p25 and 6p21.3 in cervical carcinoma: occurrence of allelic deletions in precancerous lesions. Cancer Res 2001;61:2119–23
Ryschich E, Notzel T, Hinz U, et al. Control of T-cell-mediated immune response by HLA class I in human pancreatic carcinoma. Clin Cancer Res 2005;11:498–504
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sasada, T., Azuma, K., Hirai, T. et al. Prognostic Significance of the Immediate Early Response Gene X-1 (IEX-1) Expression in Pancreatic Cancer. Ann Surg Oncol 15, 609–617 (2008). https://doi.org/10.1245/s10434-007-9669-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-007-9669-0