Abstract
Background
There is evidence that cancer is immunogenic under certain situations. IL-2 is described to stimulate an effective antitumor immune response in vitro and in vivo. The ability of cancer patients to undergo surgical resection is still the most important prognostic factor for many solid tumors, including gastric adenocarcinoma. The host immune system may be further compromised by surgical procedures leading to a generalized state of immunodepression in the post-operative period. The aim of this randomized case–control study is to evaluate the effects of pre-operative low-dose IL-2 treatment on patients with gastric adenocarcinoma who undergo surgery.
Methods
Sixty-eight patients with gastric adenocarcinoma were enrolled in the study and randomized in two groups: 36 patients were pre-treated with IL-2 and 32 underwent surgery without any treatment. Total peripheral WBC, neutrophils, CD3+ T, CD4+ T, CD8+ T and NK cells were obtained before and after surgery, at different times. Peritumoral infiltration was analyzed on all surgical specimens. Overall survival and relapse-free survival were studied with a median follow-up of 51 months.
Results
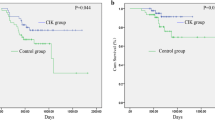
Low-dose IL-2 treatment resulted in an increase peritumoral lymphocytic and eosinophilic infiltrations and in a minor decrease in CD3+ T and CD4+ T cells after surgery (P < 0.05). A stepwise multivariate analysis revealed that overall survival and relapse-free survival were affected only by stage of tumor and age of patients.
Conclusions
According to our data low-doses of IL-2 administered pre-operatively to patients with gastric cancer activate peripheral and peri-tumoral lymphocytes but did not affect prognosis.




Similar content being viewed by others
References
Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer 2001; 37(Suppl 8):S4–66
Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12(3):354–62
Devesa SS, Blot WJ, Fraumeni JF. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998; 83:2049–53
Powell J, McConkey CC. Increasing incidence of adenocarcinoma of the gastric cardia and adjacent sites. Br J Cancer 1990; 62:440–3
Laheij RJ, Straatman H, Verbeek AL, et al. Mortality trend from cancer of the gastric cardia in The Netherlands, 1969–1994. Int J Epidemiol 1999; 28:391–5
Botterweck AA, Schouten LJ, Volovics A, et al. Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol 2000; 29:645–54
GLOBOCAN 2000: Cancer incidence, mortality and prevalence worldwide. International Agency for Research on Cancer (IARC), Lyon: IARC Press; 2001
Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base report on survival of U.S. gastric carcinoma patients treated with gastrectomy. Cancer 2000; 88:912–32
Lennard TW, Shenton BK, Borzotta A, et al. The influence of surgical operations on components of the human immune system. Br J Surg 1985; 72(10):771–6
Wakefield CH, Carey PD, Foulds S, et al. Changes in major histocompatibility complex class II expression in monocytes and T cells of patients developing infection after surgery. Br J Surg 1993; 80:205–9
Cheadle WG, Hershman MJ, Wellhausen SR, et al. HLA-DR antigen expression on peripheral blood monocytes correlates with surgical infection. Am J Surg 1991; 161:639–45
Eggermont AMM, Steller EP, Sugerbacker PH. Laparotomy enhances intraperitoneal tumour growth and abrogates the antitumor effects of interleukin-2 and lymphokine-activated killer cells. Surgery 1987; 102:71–8
Morgan D, Ruscetti F, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 1976; 193:1007–8
Smith KA. Interleukin-2: inception, impact and implications. Science 1998; 240:1169–76
Rosenberg SA, Lotze MT, Muul LM, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high dose interleukin-2 alone. N Engl J Med 1997; 316:889–97
Kovacs JA, Baseler M, Dewar RJ, et al. Increases in CD4 T lymphocytes with intermittent courses of interleukin-2 in patients with human immunodeficiency virus infection. A preliminary study. N Engl J Med 1995; 332(9):567–75
Levy Y, Capitant C, Houhou S, et al. Comparison of subcutaneous and intravenous interleukin-2 in asymptomatic HIV-1 infection: a randomised controlled trial. ANRS 048 study group. Lancet 1999; 353(9168):1923–9
Jacobson EL, Pilaro F, Smith KA. Rational interleukin 2 therapy for HIV positive individuals: daily low doses enhance immune function without toxicity. Proc Natl Acad Sci USA 1996; 93(19):10405–10
Grande C, Firvida JL, Navas V, et al. Interleukin-2 for the treatment of solid tumors other than melanoma and renal cell carcinoma. Anticancer Drugs 2006; 17(1):1–12
Repka T, Cbiorean EG, Gay J, et al. Trastuzumab and IL-2 in HER-2 positive metastatic breast cancer: a pilot study. Clin Cancer Res 2003; 9:2440–6
Fleming GF, Meropol NJ, Rosner GL, et al. A phase I trial of escalating doses of trastuzumab combined with daily subcutaneous interleukin-2: report of cancer and leukaemia group B 9661. Clin Cancer Res 2002; 8:3718–27
Dagher R, Long LM, Read EJ, et al. Pilot trial of tumor specific peptide vaccination and continuous infusion interleukin-2 in patients with recurrent Ewing sarcoma and alveolar rhabdomyosarcoma: an inter-institute NIH study. Med Pediatr Oncol 2002; 38:158–64
Fiedler W, Kruger W, Laack E, et al. A clinical trial of edrecolomab, interleukin-2 and GM-CSF in patients with advanced colorectal cancer. Oncol Rep 2001; 8:225–31
Sosman JA, Stiff P, Moss SM, et al. Pilot trial of interleukin-2 with granulocyte colony stimulating factor for the mobilization of progenitor cells in advanced breast cancer patients undergoing high-dose chemotherapy. J Clin Oncol 2001; 19:634–44
Freedman RS, Kudelka AP, Kavanagh JJ, et al. Clinical and biological effects of intraperitoneal interferon gamma and recombinant interleukin with or without tumor infiltrating lymphocytes in patients with ovarian or peritoneal carcinoma. Clin Cancer Res 2000; 6:2268–78
Tester WJ, Kim KM, Krigel RL, et al. A randomized phase II study of interleukin-2 with or without beta-interferon for patients with advanced non-small cell lung cancer. A Eastern Cooperative Oncology Group study (PZ586). Lung Cancer 1999; 25:199–206
Meehan KR, Arun B, Gehan EA, et al. Immunotherapy with interleukin-2 and alpha interferon after interleukin-2 activated hematopoietic stem cell transplantation for breast cancer. Bone Marrow Transplant 1999; 23:667–73
Luksch R, Perotti D, Cefalo G, et al. Immunomodulation in a treatment program including pre- and post- operative interleukin-2 and chemotherapy for childhood osteosarcoma. Tumori 2003; 89:263–8
Mantovani G, Maccio A, Mulas C, et al. Dose-intense phase II study of weekly cisplatin and epidoxorubicin plus medroxyprogesterone acetate and recombinant interleukin-2 in stage IIIB-IV non-small cell lung cancer. Oncol Rep 2002; 9:661–70
Yoshimura K, Hazama S, Iizuka N, et al. Hepatic arterial infusion of IL-2 and chemotherapy for unresectable liver metastases from colorectal cancer. Gan To Kagaku Ryoho 2002; 29:2117–20
Fu QG, Meng FD, Shen XD, et al. Efficacy of intraperitoneal thermochemotherapy and immunotherapy in intraperitoneal recurrence after gastrointestinal cancer resection. World J Gastroenterol 2002; 8:1019–22
Toh HC, McAfee SL, Sackstein R, et al. High-dose cyclophosphamide+carboplatin and interleukin-2 activated autologous stem cell transplantation followed by maintenance interleukin-2 therapy in metastatic breast carcinoma. A phase II study. Bone Marrow Transplant 2000; 25:19–24
Le Cesne A, Vassal G, Farace F, et al. Combination interleukin-2 and doxorubicin in advanced adult solid tumors: circumvention of doxorubicin resistance in soft tissue sarcoma? J Immunother 1999; 22:268–77
Angelini C, Bovo G, Muselli P, et al. Preoperative interleukin-2 immunotherapy in pancreatic cancer: preliminary results. Hepatogastroenterology 2006; 53(67):141–4
Romano F, Cesana G, Berselli M, et al. Biological, histological, and clinical impact of preoperative IL-2 administration in radically operable gastric cancer patients. J Surg Oncol 2004; 88(4):240–7
Kishiwada M, Kawarada Y, Taoka H, et al. Management of advanced pancreatic cancer: staging laparoscopy and immunochemotherapy. A new treatment strategy. Hepatogastroenterology 2002; 49:1704–6
Yano T, Sugio K, Yamazaki K, et al. Postoperative adjuvant adoptive immunotherapy with lymph node-LAK cells and IL-2 for pathologic stage I non-small cell lung cancer. Lung Cancer 1999; 26:143–8
Tarasov VA, Filatov MV, Kisliakova TV, et al. Combined surgical and immunotherapeutic treatment of patients with fourth stage colon cancer. Hybridoma 1999; 18:99–102
Lygidakis NJ, Berberabe AE, Spentzouris N, et al. A prospective randomized study using adjuvant locoregional chemoimmunotherapy in combination with surgery for pancreatic carcinoma. Hepatogastroenterology 1999; 45:2376–81
Melioli G, Ratto GB, Ponte M, et al. Treatment of stage IIIB non-small-cell lung cancer with surgery followed by infusion of tumor infiltrating lymphocytes and recombinant interleukin-2: a pilot study. J Immunother 1996; 19:224–30
Wang Y, Gu Q, Liu B, et al. Perspectives of SEREX-defined antigens in diagnosis and immunotherapy for gastric cancer. Cancer Biol Ther 2004; 3(9):806–11
Lissoni P, Brivio F, Ardizzoia A, et al. Subcutaneous therapy with low-dose interleukin-2 plus the neurohormone melatonin in metastatic gastric cancer patients with low performance status. Clin Tumori 1993; 79(6):401–4
Lissoni P, Barni S, Ardizzoia A, et al. Cancer immunotherapy with low-dose interleukin-2 subcutaneous administration: potential efficacy in most solid tumor histotypes by a concomitant treatment with the pineal hormone melatonin. J Biol Regul Homeost Agents 1993; 7(4):121–5
Gochi A, Orita K, Fuchimoto S, et al. The prognostic advantage of preoperative intratumoral injection of OK-432 for gastric cancer patients. Br J Cancer 2001; 84(4):443–451
Sakamoto J, Teramukai S, Nakazato H, et al. Efficacy of adjuvant immunochemotherapy with OK-432 for patients with curatively resected gastric cancer: a meta-analysis of centrally randomized controlled clinical trials. J Immunother 2002; 25(5):405–12
Brivio F, Fumagalli L, Lissoni P, et al. Pre-operative immunoprophylaxis with interleukin-2 may improve prognosis in radical surgery for colorectal cancer stage B–C. Anticancer Res 2006; 26(1B):599–603
Brivio F, Lissoni P, Gilardi R, et al. Abrogation of surgery-induced decline in circulating dentritic cells by subcutaneous preoperative administration of IL-2 in operable cancer patients. J Biol Regul Homeost Agents 2000; 14(3):200–3
Brivio F, Lissoni P, Alderi G, et al. Preoperative interleukin-2 subcutaneous immunotherapy may prolong the survival time in advanced colorectal cancer patients. Oncology 1996; 53:263–8
Ropponen KM, Eskilinen MJ, Lipponen PK, et al. Prognostic value of tumor infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol 1997; 182:318–24
Cerea K, Romano F, Bravo AF, et al. Phase IB study on prevention of surgery-induced immunodeficiency with preoperative administration of low-dose subcutaneous interleukin-2 in gastric cancer patients. J Surg Oncol 2001; 78(1):32–7
Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 2006; 6:295–307
Shevach EM. Fatal attraction: tumours beckon regulatory T cells. Nat Med 2004; 10:900–1
Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999; 17(7):2105–16
Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 1995; 13:688–96
Fisher RI, Coltman CA Jr, Doroshow JH, et al. Metastatic renal cancer treated with interleukin-2 and lymphokine-activated killer cells. A phase II clinical trial. Ann Intern Med 1988; 108(4):518–23
Rosenberg SA, Yang JC, White DE, et al. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2. Ann Surg 1998; 228:319
Fisher RI, Rosenberg SA, Sznol M, et al. High-dose aldesleukin in renal cell carcinoma: long term survival update. Cancer J Sci Am 1997; 3:S70–2
Cesana GC, DeRaffele G, Cohen S, et al. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol 2006; 24:1169–77
Kodera Y, Fujiwara M, Koike M, et al. Chemotherapy as a component of multimodal therapy for gastric carcinoma. World J Gastroenterol 2006; 12(13):2000–5
Wang E, Panelli MC, Monsurro V, et al. A global approach to tumor immunology. Cell Mol Immunol 2004; 1(4):256–65
Bovo G, Brivio F, Brenna A, et al. Pre-operative interleukin-2 immunotherapy induces eosinophilic infiltration in colorectal neoplastic stroma. Pathologica 1995; 87(2):135–8
Silberstein DS, Schoof DD, Rodrick ML, et al. Activation of eosinophils in cancer patients treated with IL-2 and IL-2-generated lymphokine-activated killer cells. J Immunol 1989; 142(6):2162–7
Villegas FR, Coca S, Villarrubia VG, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer 2002; 35:23–8
Friedl J, Stift A, Paolini P, et al. Tumor antigen pulsed dendritic cells enhance the cytolityc activity of tumor-infiltrating lymphocytes in human hepatocellular cancer. Cancer Biother Radiopharm 2000; 15:477–86
Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348(3):203–13
Coca S, Perez-Piqueras J, Martinez D, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer 1997; 79:2320–8
Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353:2654–66
Vesalainen S, Lipponen P, Taljia M, et al. Histological grade, perineural infiltration, tumor-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer 1994; 30:1797–1803
Setala LP, Kosma VM, Marin S, et al. Prognostic factors in gastric cancer: the value of vascular invasion, mitotic rate and lymphoplasmacytic infiltration. Br J Cancer 1996; 74:766–772
Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 2003; 21(16):3127–32
Acknowledgments
The authors are indebted to Dr Howard Kaufman, Edwin C. and Anne K. Weiskopf Associate Professor of Clinical Surgical Oncology at Columbia University College of Physicians & Surgeons (New York, NY, USA), for his scientific support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cesana, G.C., Romano, F., Piacentini, G. et al. Low-dose Interleukin-2 Administered Pre-operatively to Patients with Gastric Cancer Activates Peripheral and Peritumoral Lymphocytes But Does Not Affect Prognosis. Ann Surg Oncol 14, 1295–1304 (2007). https://doi.org/10.1245/s10434-006-9239-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-006-9239-x