Abstract
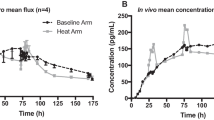
Application of heat (hyperthermic conditions) on skin is known to enhance drug transfer and facilitate skin penetration of molecules. The aim of this work was to study the effect of hyperthermia on the drug release and skin permeation from nicotine transdermal patches. The drug release and skin permeation were characterized by in vitro release test and in vitro permeation test. The temperature was maintained at 32 °C as control (simulating normal physiological skin temperature) and 42 °C as hyperthermia condition. The in vitro release test was carried out using USP apparatus 5-Paddle over disk method for a transdermal patch. Skin permeation study was carried out across porcine skin using the flow through cells (PermeGear, Inc.) with an active diffusion area of 0.94 cm2. Mechanistic studies (parameters such as partition coefficient, TEWL and electrical resistivity) were also performed to understand the mechanisms involved in determining the influence of hyperthermia on drug delivery from transdermal patches of nicotine. The rate and extent of drug release from nicotine patch was not significantly different at two temperatures (Cumulative release after 12 h was 43.99 ± 3.29% at 32 °C and 53.70 ± 5.14% at 42 °C). Whereas, in case of in vitro permeation studies, the nicotine transdermal permeation flux for patch was threefold higher at 42 °C (100.1 ± 14.83 μg/cm2/h) than at 32 °C (33.3 ± 14.83 μg/cm2/h). The mechanistic studies revealed that the predominant mechanism of enhancement of drug permeation by hyperthermia condition is by the way of increasing the skin permeability. There is a potential concern of dumping of higher dose of nicotine via transdermal route.





Similar content being viewed by others
Abbreviations
- HPLC:
-
High performance liquid chromatography
- UV:
-
Ultraviolet
- PBS:
-
Phosphate buffer saline
- TEWL:
-
Trans epidermal water loss
- RL :
-
Load resistor
- V O :
-
Voltage drop across entire circuit
- V ep :
-
Voltage drop across skin
- R ep :
-
Skin resistance
- USP:
-
United States Pharmacopeia
References
Petersen KK, Rousing ML, Jensen C, Arendt-Nielsen L, Gazerani P. Effect of local controlled heat on transdermal delivery of nicotine. Int J Physiol Pathophysiol Pharmacol. 2011;3(3):236–42.
Vanakoski J, Seppälä T, Sievi E, Lunell E. Exposure to high ambient temperature increases absorption and plasma concentrations of transdermal nicotine. Clin Pharmacol Ther. 1996 Sep 1;60(3):308–15.
Rose PG, Macfee MS, Boswell MV. Fentanyl transdermal system overdose secondary to cutaneous hyperthermia. Anesth Analg. 1993;77:390–1.
Vanakoski J, Seppala T. Heat exposure and drugs. A review of the effects of hyperthermia on pharmacokinetics. Clin Pharmacokinet. 1998;34:311–22.
U.S. Food and Drug Administration. FDA issues second safety warning on fentanyl skin patch. Deaths and serious injuries from improper use; 2007; Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm109046.htm.
Newshan G. Heat-related toxicity with the fentanyl transdermal patch. J Pain Symptom Manag. 1998;16:277–8.
Barkve TF, Langseth-Manrique K, Bredesen JEandGjesdal K. Increased uptake of transdermal glyceryl trinitrate during physical exerciseand during high ambient temperature. Am Heart J. 1986;112:537–41.
Sindali K, Sherry K, Sen S, Dheansa B. Life-threatening coma and full-thickness sunburn in a patient treated with transdermal fentanyl patches: a case report. J Med Case Rep. 2012;6:220.
Shin SH, Ghosh P, Newman B, Hammell DC, Raney SG, Hassan HE, et al. On the road to development of an in vitro permeation test (IVPT) model to compare heat effects on transdermal delivery systems: exploratory studies with nicotine and fentanyl. Pharm Res. 2017;34(9):1817–30. https://doi.org/10.1007/s11095-017-2189-0.
Moore KT, Sathyan G, Richarz U, Natarajan J, Vandenbossche J. Randomized 5-treatment crossover study to assess the effects of external heat on serum fentanyl concentrations during treatment with transdermal fentanyl systems. J Clin Pharmacol. 2012;52:1174–85.
Forster M, Bolzinger MA, Fessi H, Briancon S. Topical delivery of cosmetics and drugs. Molecular aspects of percutaneous absorption and delivery. Eur J Dermatol. 2009;19:309–23.
Paudel KS, Milewski M, Swadley CL, Brogden NK, Ghosh P, Stinchcomb AL. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv. 2010;1:109–31.
Wiedersberg S, Guy RH. Transdermal drug delivery: 30+ years of war and still fighting! J Control Release. 2014;190:150–6.
Park J-H, Lee J-W, Kim Y-C, Prausnitz MR. The effect of heat on skin permeability. Int J Pharm. 2008;359:94–103.
Shomaker TS, Zhang J, Ashburn MA. Assessing the impact of heat on the systemic delivery of fentanyl through the transdermal fentanyl delivery system. Pain Med. 2000;1:225–30.
Thong HY, Zhai H, Maibach HI. Percutaneous penetration enhancers: an overview. Skin Pharmacol Physiol. 2007;20:272–82.
Hafeez F, Chiang A, Hui X, Maibach H. Role of partition coefficients in determining the percutaneous penetration of salicylic acid and formaldehyde under varying occlusion durations. Drug Dev Ind Pharm. 2014;40(10):1395–401. https://doi.org/10.3109/03639045.2013.828218.
Manda P, Angamuthu M, Hiremath SR, Raman V, Murthy SN. Iontophoretic drug delivery for the treatment of scars. J Pharm Sci. 2014;103(6):1638–42. https://doi.org/10.1002/jps.23946.
Noubigh A, Mgaidi A, Abderrabba M. Temperature effect on the distribution of some phenolic compounds: an experimental measurement of 1-octanol/water partition coefficients. J Chem Eng Data. 2010;55(1):488–91. https://doi.org/10.1021/je900271h.
Noubigh A, Abderrabba M, Provost E. Temperature and salt addition effects on the solubility behaviour of some phenolic compounds in water. J Chem Thermodyn. 2007;39:297–303.
Plessis J d, Stefaniak A, Eloff F, John S, Agner T, Chou T, et al. International guidelines for the in vivo assessment of skin properties in non-clinical settings: part 2. Transepidermal water loss and skin hydration. Skin Res Technol. 2013;19(3):265–78. https://doi.org/10.1111/srt.12037.
Berardesca E, Maibach HI. Racial differences in sodium lauryl sulphate induced cutaneous irritation: black and white. Contact Dermatitis. 1988a;18:65–70.
Mathias CGT, Wilson D, Maibach HI. Transepidermal water loss as a function of skin temperature. J Invest Dermatol. 1981;77:219–20.
Rogiers V, EEMCO Group. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol Appl Ski Physiol. 2001;14(2):117–28. https://doi.org/10.1159/000056341.
Berardesca E, Maibach HI. Sodiumlauryl- sulphate-induced cutaneous irritation: comparison of white and Hispanic subjects. Contact Dermatitis. 1988b;19:136–40.
Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48:S139–42.
Narasimha Murthy S, Sen A, Zhao Y, Hui SW. Temperature influences the postelectroporation permeability state of the skin. J Pharm Sci. 2004;93(4):908–15. https://doi.org/10.1002/jps.20016.
Prausnitz MR. The effects of electric current applied to skin: a review for transdermal drug delivery. Adv Drug Deliv Rev. 1996;18(3):395–425. https://doi.org/10.1016/0169-409X(95)00081-H.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Panda, A., Sharma, P.K. & Narasimha Murthy, S. Effect of Mild Hyperthermia on Transdermal Absorption of Nicotine from Patches. AAPS PharmSciTech 20, 77 (2019). https://doi.org/10.1208/s12249-019-1299-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1299-x