Abstract
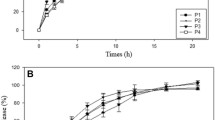
Optimization of a lyophilized fast-disintegrating tablet (LFDT) formulation containing naratriptan hydrochloride, an antimigraine drug, was the foremost objective of the study, aiming in achieving fast headache pain relief. The Design-Expert® v10 software was used to generate formulations using D-optimal mixture design with four components: gelatin (X1), hydrolyzed gelatin (X2), glycine (X3), and mannitol (X4) of total solid material (TSM) w/w. The effect of the relative proportion of each component was determined on friability (Y1), hardness (Y2), and in vitro disintegration time (Y3), which was then applied for formulation optimization. In addition, their effect on tablet porosity was determined via scanning electron microscopy (SEM). Drug-excipient interaction was evaluated using differential scanning calorimetry (DSC). A comparative dissolution study against the conventional tablets was studied. Accelerated stability study was carried out in (Al/Al) and (Al/PVC) blister packs. An in vivo pharmacokinetic study was carried out to compare the optimized formulation and the conventional tablets. The optimized formulation’s responses were 0.30%, 3.4 kg, and 6.12 s for Y1, Y2, and Y3, respectively. No drug-excipient interaction was specified via DSC. The optimized formulation exhibited porous structure as determined via SEM. Dissolution study demonstrated complete dissolution within 1.5 min. Study indicated stability for 78 months in (Al/Al) blister packs. In vivo pharmacokinetic study demonstrated that Cmax, AUClast, and AUCinf were significantly higher for the developed formulation. As well, the Tmax was 1 h earlier than that of convenient tablet. An LFDT would achieve a faster onset of action for naratriptan compared to other formulations.






Similar content being viewed by others
Change history
16 July 2018
During the production process, an editorial error occurred where the typesetter placed the ± symbol on the right side of the values in Table IV, whereas the symbol should be placed on the left side. The original article has been corrected.
References
IHS HCCot. The international classification of headache disorders, 3rd edition. Cephalalgia. 2013;33(9):629–808.
Aggarwal M, Puri V, Puri S. Serotonin and CGRP in migraine. Ann Neurosci. 2012;19(2):88–94.
Ahn AH, Basbaum AI. Where do triptans act in the treatment of migraine? Pain. 2005;115(1–2):1–4.
Bigal ME, Bordini CA, Antoniazzi AL, Speciali JG. The triptan formulations: a critical evaluation. Arq Neuropsiquiatr. 2003;61(2-A):313–20.
Jhee SS, Shiovitz T, Crawford AW, Cutler NR. Pharmacokinetics and pharmacodynamics of the triptan antimigraine agents. A comparative review. Clin Pharmacokinet. 2001;40:189–205.
Newman L, Mannix LK, Landy S, Silberstein S, Lipton RB, Pait Putnam DG, et al. Naratriptan as short-term prophylaxis of menstrually associated migraine: a randomized, double-blind, placebo-controlled study. Headache. 2001;41:248–56.
Fu Y, Yang S, Jeong SH, Kimura S, Park K. Orally fast disintegrating tablets: developments, technologies, taste-masking and clinical studies. Crit Rev Ther Drug Carrier Syst. 2004;21(6):433–75.
FDA. Guidance for industry: orally disintegrating tablets. 2008.
Parkash V, Maan S, Deepika, Yadav SK, Hemlata, Jogpal V. Fast disintegrating tablets: opportunity in drug delivery system. J Adv Pharm Technol Res. 2011;2(4):223–35.
AlHusban F, Perrie Y, Mohammed AR. Preparation, optimisation and characterisation of lyophilised rapid disintegrating tablets based on gelatin and saccharide. Curr Drug Deliv. 2010;7:65–75.
AlHusban F, Perrie Y, Mohammed AR. Formulation and characterisation of lyophilised rapid disintegrating tablets using amino acids as matrix forming agents. Eur J Pharm Biopharm. 2010;75(2):254–62.
Mahmoud AA, Salah S. Fast relief from migraine attacks using fast-disintegrating sublingual zolmitriptan tablets. Drug Dev Ind Pharm. 2012;38(6):762–9.
Zayed R, Kamel AO, Shukr M, El-shamy A-h. An in vitro and in vivo comparative study of directly compressed solid dispersions and freeze dried sildenafil citrate sublingual tablets for management of pulmonary arterial hypertension. Acta Pharma. 2012;62:411–32.
Jaipal A, Pandey M, Charde S, Raut P, Prasanth K, Prasad R. Effect of HPMC and mannitol on drug release and bioadhesion behavior of buccal discs of buspirone hydrochloride: in-vitro and in-vivo pharmacokinetic studies. Saudi Pharm J. 2015;23(3):315–26.
Shaikh M, Derle ND, Bhamber R. Permeability enhancement techniques for poorly permeable drugs: a review. J Appl Pharm Sci. 2012;2(6):34–9.
FDA. Guidance for industry: Q8(R2) pharmaceutical development 2009.
(EMA) EMA. Note for guidance on development pharmaceutics. 1998.
Convention USP. United States Pharmacopeia 35. 2011.
35 USP. [1216] Tablet friability. 2011:867–8.
35 USP. [1217] Tablet breaking force. 2011:868–70.
35 USP. [701] Disintegration. 2011:293–5.
Koland M, Charyulu RN, Vijayanarayana K, Prabhu P. In vitro and in vivo evaluation of chitosan buccal films of ondansetron hydrochloride. Int J Pharm Investig. 2011;1(3):164–71.
Marques MR, Loebenberg R, Almukainzi M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011;18(3):15–28.
Yeom DW, Song YS, Kim SR, Lee SG, Kang MH, Lee S, et al. Development and optimization of a self-microemulsifying drug delivery system for ator vastatin calcium by using D-optimal mixture design. Int J Nanomedicine. 2015;10:3865–78.
35 USP. [905] Uniformity of dosage units. 2011:420–3.
35 USP. Naratriptan tablets. 2011:4002–3.
Ahuja N, Katare OP, Singh B. Studies on dissolution enhancement and mathematical modeling of drug release of a poorly water-soluble drug using water-soluble carriers. Eur J Pharm Biopharm. 2006;65:26–38.
FDA. Guidance for industry: dissolution testing of immediate release solid oral dosage forms. 1997
ICH. Stability testing of new drug substances and products Q1A(R2). 2003
Wilson D, Wren S, Reynolds G. Linking dissolution to disintegration in immediate release tablets using image analysis and a population balance modelling approach. Pharm Res. 2012;29(1):198–208.
Azarmi S, Roa W, Löbenberg R. Current perspectives in dissolution testing of conventional and novel dosage forms. Int J Pharm. 2007;328(1):12–21.
ICH. Validation of analytical procedures. 2005.
O’Donnell PB, Bokser AD. Stability of pharmaceutical products. In: Gennaro AR, editor. Remington: the science and practice of pharmacy. 21st ed. Philadelphia: Lippincott Williams & Wilkins; 2005. p. 1025.
Yoshioka S, Stella VJ. Stability of dosage forms. Stability of drugs and dosage forms, vol. 2000. 1st ed. Berlin: Springer. p. 151–86.
Ferrari A, Sternieri E, Tiraferri I, Neri L. Why pharmacokinetic differences among oral triptans have little clinical importance: a comment. J Headache Pain. 2011;12:5–12.
(EMA) EMA. Guideline on the investigation of bioequivalence. 2010.
(EMA) EMA. Guideline on bioanalytical method validation. 2011.
Gohel M, Patel M, Amin A, Agrawal R, Dave R, Bariya N. Formulation design and optimization of mouth dissolve tablets of nimesulide using vacuum drying technique. AAPS PharmSciTech. 2004;5(3):1–6.
Hawe A, Frieß W. Impact of freezing procedure and annealing on the physico-chemical properties and the formation of mannitol hydrate in mannitol–sucrose–NaCl formulations. Eur J Pharm Biopharm. 2006;64:316–25.
Fukami J, Yonemochi E, Yoshihashi Y, Terada K. Evaluation of rapidly disintegrating tablets containing glycine and carboxymethylcellulose. Int J Pharm. 2006;310(1–2):101–9.
Sunada H, Bi Y. Preparation, evaluation and optimization of rapidly disintegrating tablets. Powder Technol. 2002;122:188–98.
Eysturskarð J. Mechanical properties of gelatin gels; effect of molecular weight and molecular weight distribution [PhD thesis]: Norwegian University of Science and Technology; 2010.
Jinichi F, Ozawa A, Yoshihashi Y, Yonemochi E, Terada K. Development of fast disintegrating compressed tablets using amino acid as disintegration accelerator: evaluation of wetting and disintegration of tablet on the basis of surface free energy. Chem Pharm Bull. 2005;53(13):1536–9.
Ahmed IS, Aboul-Einien MH. In vitro and in vivo evaluation of a fast-disintegrating lyophilized dry emulsion tablet containing griseofulvin. Eur J Pharm Sci. 2007;32:58–68.
Ahmed IS, Shamma RN, Shoukri RA. Development and optimization of lyophilized orally disintegrating tablets using factorial design. Pharm Dev Technol. 2013;18(4):935–43.
Shoukri RA, Ahmed IS, Shamma RN. In vitro and in vivo evaluation of nimesulide lyophilized orally disintegrating tablets. Eur J Pharm Biopharm. 2009;763:162–71.
Patel P, Ahir K, Patel V, Manani L, Patel C. Drug-excipient compatibility studies: first step for dosage form development. Pharm Innov J. 2015;4:14–20.
Ujhelyiová A, Marcinèin A, Legéò J. DSC analysis of polypropylene-low density polyethylene blend fibres. Fibres Text East Eur. 2005;13(5 (53)):129–32.
Sharma D, Singh G, Kumar D, Singh M. Formulation development and evaluation of fast disintegrating tablets of salbutamol sulphate, cetirizine hydrochloride in combined pharmaceutical dosage form: a new era in novel drug delivery for pediatrics and geriatrics. J Drug Deliv. 2015;2015:1–10.
Allinson JG, Dansereau RJ, Sakr A. The effects of packaging on the stability of a moisture sensitive compound. Int J Pharm. 2001;221:49–56.
Ghosh T, Ghosh A, Prasad D. A review on new generation orodispersible tablets and its future prospective. Int J Pharm Pharm Sci. 2011;3(1):1–7.
FDA. Guidance for industry: statistical approaches to establishing bioequivalence. 2001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
The original version of this article was revised: During the production process, an editorial error occurred where the typesetter placed the ± symbol on the right side of the values in Table IV, whereas the symbol should be placed on the left side. Also in Table IV, the AI/PVC was changed from 3.39d to 3.32d in month 5 after publication. In Table IV and Table V the < sign was omitted during the production process.
Rights and permissions
About this article
Cite this article
Omar, S.M., AbdAlla, F.I. & Abdelgawad, N.M. Preparation and Optimization of Fast-Disintegrating Tablet Containing Naratriptan Hydrochloride Using D-Optimal Mixture Design. AAPS PharmSciTech 19, 2472–2487 (2018). https://doi.org/10.1208/s12249-018-1061-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-1061-9