Abstract
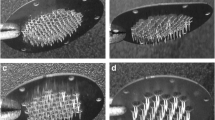
The present study was aimed to investigate the effect of salient microneedle (MN) geometry parameters like length, density, shape and type on transdermal permeation of rizatriptan (RIZ). Studies were carried out using two types of MN devices viz. AdminPatch® arrays (ADM) (0.6, 0.9, 1.2 and 1.5 mm lengths) and laboratory-fabricated polymeric MNs (PMs) of 0.6 mm length. In the case of the PMs, arrays were applied three times at different places within a 1.77-cm2 skin area (PM-3) to maintain the MN density closer to 0.6 mm ADM. Histological studies revealed that PM, owing to their geometry/design, formed wider and deeper microconduits when compared to ADM of similar length. Approximately 4.9- and 4.2-fold increases in the RIZ steady-state flux values were observed with 1.5 mm ADM and PM-3 applications when compared to the passive studies. A good correlation between different dimensionless parameters like the amount of RIZ permeated (C t /C s), thickness (h/L) and surface area (S a/L 2) of the skin was observed with scaling analyses. Numerical simulations provided further information regarding the distribution of RIZ in MN-treated skin after application of different MNs. Overall, the study suggests that MN application enhances the RIZ transdermal permeation and the geometrical parameters of MNs play an important role in the degree enhancement.










Similar content being viewed by others
References
Brown MB, Gary PM, Stuart AJ, Franklin KA. Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv. 2006;13:175–87.
Prausnitz MR, Robert L. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–8.
Cheung K, Das DB. Microneedles for drug delivery: trends and progress. Drug Deliv. 2014;23:1–7.
Prausnitz MR, Samir M, Robert L. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3:115–24.
Ritesh K, Anil P. Modified transdermal technologies: breaking the barriers of drug permeation via the skin. Trop J Pharm Res. 2007;6:633–44.
Han T, Das DB. Potential of combined ultrasound and microneedles for enhanced transdermal drug permeation: a review. Eur J Pharm Biopharm. 2015;89:312–28.
Silberstein SD. Migraine symptoms: results of a survey of self-reported migraineurs. Headache. 1995;35:387–96.
Rapoport AM, Tepper SJ, Sheftell FD, Kung E, Bigal ME. Which triptan for which patient? Neurol Sci. 2006;27:S123–9.
Marcelo EB, Carlos AB, Ana LA, José G. The triptan formulations—a critical evaluation. Speciali Arq Neuropsiquiatr. 2003;61:313–20.
Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24:585–94.
Jeong W, Lee A, Jung-Hwan PB, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29:2113–24.
Zhou CP, Liu YL, Wang HL, Zhang PX, Zhang JL. Transdermal delivery of insulin using microneedle rollers in vivo. Int J Pharm. 2010;39:127–33.
Nalluri BN, Sai Sri Anusha V, Bramhini SR, Amulya J, Sultana AS, Teja UC, et al. In vitro skin permeation enhancement of sumatriptan by microneedle application. Curr Drug Deliv. 2015;12:761–9.
Gomaa YA, Morrow DI, Garland MJ, Donnelly RF, El-Khordagui LK, Meidan VM. Effects of microneedle length, density, insertion time and multiple applications on human skin barrier function: assessments by transepidermal water loss. Toxicol In Vitro. 2010;24:1971–8.
Attia UM, Marsona S, Alcock JR. Micro-injection moulding of polymer microfluidic devices. Microfluid Nanofluid. 2009;7:1–28.
Nair KJ, Whiteside BR, Grant C, Patel R, Tuinea-Bobe C, Norris K, et al. Investigation of plasma treatment on micro-injection moulded microneedle for drug delivery. Pharmaceutics. 2015;7:471–85.
Al-Qallaf B, Das DB, Mori D, Cui Z. Modelling transdermal delivery of high molecular weight drugs from microneedle systems. Phil Trans R Soc A. 2007;365:2951–67.
Leeladurga V, Teja UC, Sultana SA, Sudeep K, Anusha VS, Han T, et al. Application of microneedle arrays for enhancement of transdermal permeation of insulin: in vitro experiments, scaling analyses and numerical simulations. AAPS PharmSciTech. 2015;29:1–8.
Han T, Das DB. A new paradigm for numerical simulation of microneedle-based drug delivery aided by histology of microneedle-pierced skin. J Pharm Sci. 2015;104:1993–2007.
Yuzhakov VV, Yuzhakov Vadim V. Microneedle array, patch, and applicator for transdermal drug delivery. United States Patent US 7,658,728. 2010.
Acknowledgements
The authors are thankful to Mylan Pharmaceuticals India Ltd, Hyderabad, for providing a gift sample of RIZ; to Dr. Naveen, Department of Pathology, Dr. Pinnamaneni Siddhartha Institute of Medical Sciences and Research Foundation, Vijayawada, for providing the required facilities for taking histological sections of skin samples; and also to the Siddhartha Academy of General and Technical Education, Vijayawada, for providing the necessary facilities to carry out the research work. The authors also extend their sincere thanks to DST, Ministry of Science and Technology, Govt. of India, and the British Council, London, UK, for funding this research work under the DST-UKIERI scheme (DST/INT/UK/P-60/2014).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Guest Editors: Dr. Z Ahmad and Prof. M Edirisinghe
Rights and permissions
About this article
Cite this article
Uppuluri, C., Shaik, A., Han, T. et al. Effect of Microneedle Type on Transdermal Permeation of Rizatriptan. AAPS PharmSciTech 18, 1495–1506 (2017). https://doi.org/10.1208/s12249-016-0702-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0702-0