Abstract
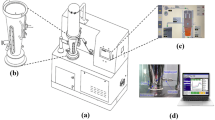
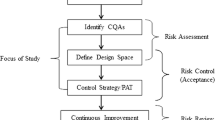
Current endeavor was aimed towards monitoring percent weight build-up during functional coating process on drug-layered pellets. Near-infrared (NIR) spectroscopy is an emerging process analytical technology (PAT) tool which was employed here within quality by design (QbD) framework. Samples were withdrawn after spraying every 15-Kg cellulosic coating material during Wurster coating process of drug-loaded pellets. NIR spectra of these samples were acquired using cup spinner assembly of Thermoscientific Antaris II, followed by multivariate analysis using partial least squares (PLS) calibration model. PLS model was built by selecting various absorption regions of NIR spectra for Ethyl cellulose, drug and correlating the absorption values with actual percent weight build up determined by HPLC. The spectral regions of 8971.04 to 8250.77 cm−1, 7515.24 to 7108.33 cm−1, and 5257.00 to 5098.87 cm−1 were found to be specific to cellulose, where as the spectral region of 6004.45 to 5844.14 cm−1was found to be specific to drug. The final model gave superb correlation co-efficient value of 0.9994 for calibration and 0.9984 for validation with low root mean square of error (RMSE) values of 0.147 for calibration and 0.371 for validation using 6 factors. The developed correlation between the NIR spectra and cellulose content is useful in precise at-line prediction of functional coat value and can be used for monitoring the Wurster coating process.






Similar content being viewed by others
References
Jones D. Development, optimization, and scale-up of process parameters: wurster coating. In: Qiu Y, Chen Y, Zhang G, Liu L, Porter W, editors. Developing solid oral dosage forms [Internet]. First edit. Elsevier Inc.; 2009. 798–816.
Wurster DE. Landmark article: air-suspension technique of coating drug particles. J Am Pharm Assoc. 2012;52(5):707–10.
De Souza LFG, Nitz M, Taranto OP. Film coating of nifedipine extended release pellets in a fluid bed coater with a wurster insert. Biomed Res Int Hindawi PublCorp. 2014;2014:1–11.
Bhattacharjya S, Wurster DE. Investigation of the drug release and surface morphological properties of film-coated pellets, and physical, thermal and mechanical properties of free films as a function of various curing conditions. AAPS PharmSciTech. 2008;9(2):449–57.
Iida K, Todo H, Okamoto H, Danjo K, Leuenberger H. Preparation of dry powder inhalation with lactose carrier particles surface-coated using a Wurster fluidized bed. Chem Pharm Bull (Tokyo). 2005;53(4):431–4.
Chan LW, Tang ESK, Heng PWS. Comparative study of the fluid dynamics of bottom spray fluid bed coaters. AAPS PharmSciTech. 2006;7(2):E37.
Ozturk AG, Ozturk SS, Palsson BO, Wheatley TA, Dressman JB. Mechanism of release from pellets coated with an ethylcellulose-based film. J Control Release. 1990;14(3):203–13.
FDA. Quality by design for ANDAs: an example for modified release dosage forms. 2011. p. 1–161. Available from: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/UCM286595.pdf Accessed 23 May 2016.
ICH/USFDA. Pharmaceutical Quality System Q10. 2009. http://www.fda.gov/downloads/Drugs/…/Guidances/ucm073517.pdf Accessed 23 May 2016.
ICH Expert Working Group. Pharmaceutical Development Q8. Vol. 8. 2009. p. 1–28. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdf Accessed 23 May 2016.
ICH Expert Working Group. Quality Risk Management Q9. 2005 p. 1–23. http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Quality+Risk+Management+Q9#0\ http://www.cls.co.at/downloads/ich-q9---quality-risk-management.pdf Accessed 23 May 2016.
FDA. Pharmaceutical CGMPs for the 21s Century - A risk-based approach. 2004. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/Manufacturing/QuestionsandAnswersonCurrentGoodManufacturingPracticescGMPforDrugs/UCM071836. Accessed 23 May 2016.
FDA. Guidance for industry PAT — a framework for innovative pharmaceutical development, manufacuring, and quality assurance. 2004 [cited 2016 May 23]. p.16. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070305.pdf.
Simon LL, Pataki H, Marosi G, Meemken F, Hungerbu K, Baiker A, et al. Assessment of recent Process Analytical Technology (PAT) trends: a multiauthor review. Orig Process Res Dev. 2014;19:3–62.
Avalle P, Pollitt MJ, Bradley K, Cooper B, Pearce G, Djemai A, et al. Development of Process Analytical Technology (PAT) methods for controlled release pellet coating. Eur J Pharm Biopharm. 2014;87(2):244–51.
Menezes JC, Ferreira AP, Rodrigues LO, Brás LP, Alves TP. Chemometrics role within the PAT context: examples from primary pharmaceutical manufacturing. Compr Chemom. 2010;4:313–55.
Alshihabi F, Vandamme T, Betz G. Focused beam reflectance method as an innovative (PAT) tool to monitor in-line granulation process in fluidized bed. Pharm Dev Technol. 2013;18(1):73–84.
Markl D, Wahl PR, Menezes JC, Koller DM, Kavsek B, Francois K, et al. Supervisory control system for monitoring a pharmaceutical hot melt extrusion process. AAPS PharmSciTech. 2013;14(3):1034–44.
Hohl R, Scheibelhofer O, Stocker E, Behzadi SS, Haack D, Koch K, et al. Monitoring of a hot melt coating process via a novel multipoint near-infrared spectrometer. AAPS PharmSciTech. 2016.
Muller J, Brock D, Knop K, Axel Zeitler J, Kleinebudde P. Prediction of dissolution time and coating thickness of sustained release formulations using Raman spectroscopy and terahertz pulsed imaging. Eur J Pharm Biopharm. 2012;80(3):690–7.
Akseli I, Cetinkaya C. Acoustic testing and characterization techniques for pharmaceutical solid dosage forms. J Pharm Innov. 2008;3(4):216–26.
Luypaert J, Massart DL, Vander Heyden Y. Near-infrared spectroscopy applications in pharmaceutical analysis. Talanta. 2007;72(3):865–83.
Guenard R, Thurau G. Implementation of process analytical technologies. In: Bakeev KA, editor. Process analytical technology: spectroscopic tools and implementation strategies for the chemical and pharmaceutical industries. first. Noida: Blackwell Publishing; 2005. p. 17–36.
Peng T, Huang Y, Mei L, Wu L, Chen L, Pan X, et al. Study progression in application of process analytical technologies on film coating. Asian J Pharm Sci Elsevier Ltd. 2014;10(3):176–85.
Lee MJ, Seo DY, Lee HE, Wang IC, Kim WS, Jeong MY, et al. In line NIR quantification of film thickness on pharmaceutical pellets during a fluid bed coating process. Int J Pharm. 2011;403(1-2):66–72.
Kuriyama A, Ozaki Y. Assessment of active pharmaceutical ingredient particle size in tablets by Raman chemical imaging validated using polystyrene microsphere size standards. AAPS PharmSciTech. 2014;15(2):375–87.
Yokoyama M, Tourigny M, Moroshima K, Suzuki J, Sakai M, Iwamoto K, et al. A novel rapid quantitative analysis of drug migration on tablets using laser induced breakdown spectroscopy. Chem Pharm Bull. 2010;58(11):1521–4.
Hudovornik G, Korasa K, Vrečer F. A study on the applicability of in-line measurements in the monitoring of the pellet coating process. Eur J Pharm Sci. 2015;75:160–8.
Närvänen T. Particle size determination during fluid bed granulation. Finland: University of Helsinki; 2009.
Markl D, Zettl M, Hannesschläger G, Sacher S, Leitner M, Buchsbaum A, et al. Calibration-free in-line monitoring of pellet coating processes via optical coherence tomography. Chem Eng Sci. 2014. doi:10.1016/j.ces.2014.05.049.
Tabasi SH, Fahmy R, Bensley D, O’Brien C, Hoag SW. Quality by design, part I: application of NIR spectroscopy to monitor tablet manufacturing process. J Pharm Sci. 2008;97(9):4040–51.
Gupta A, Peck GE, Miller RW, Morris KR. Real-time near-infrared monitoring of content uniformity, moisture content, compact density/tensile strength, and young’s modulus of roller compacted powder blends. J Pharm Sci. 2005;94(7):1589–97.
Papp MK, Pujara CP, Pinal R. Monitoring of high-shear granulation using acoustic emission: predicting granule properties. J Pharm Innov. 2008;3(2):113–22.
Reich G. Near-infrared spectroscopy and imaging: basic principles and pharmaceutical applications. Adv Drug Deliv Rev. 2005;57(8):1109–43.
Silva AFT, Burggraeve A, Denon Q, Van Der Meeren P, Sandler N, Van Den Kerkhof T. Particle sizing measurements in pharmaceutical applications: comparison of in-process methods versus off-line methods. Eur J Pharm Biopharm. 2013;85(3 PART B):1006–18.
Jamrógiewicz M. Application of the near-infrared spectroscopy in the pharmaceutical technology. J Pharm Biomed Anal. 2012;66:1–10.
Lin H, May RK, Evans MJ, Zhong S, Gladden LF, Shen Y, et al. Impact of processing conditions on inter-tablet coating thickness variations measured by terahertz in-line sensing. J Pharm Sci. 2015;104(8):2513–22.
Moes JJ, Ruijken MM, Gout E, Frijlink HW, Ugwoke MI. Application of process analytical technology in tablet process development using NIR spectroscopy: blend uniformity, content uniformity and coating thickness measurements. Int J Pharm. 2008;357(1–2):108–18.
Smith-goettler BB, Gendron CM, Macphail N, Meyer RF, Phillips JX. NIR monitoring of a hot-melt extrusion process. Spectroscopy. 2014 [cited 2016 Jul 5]. p. 1–8. Available from: http://www.spectroscopyonline.com/nir-monitoring-hot-melt-extrusion-process.
Andersson M, Folestad S, Gottfries J, Johansson MO, Josefson M, Wahlund KG. Quantitative analysis of film coating in a fluidized bed process by in-line NIR spectrometry and multivariate batch calibration. Anal Chem. 2000;72(9):2099–108.
Burgbacher J, Wiss J. Industrial applications of online monitoring of drying processes of drug substances using NIR. Org Process Res Dev. 2008;12(2):235–42.
Wildfong PLD, Samy AS, Corfa J, Peck GE, Morris KR. Accelerated fluid bed drying using NIR monitoring and phenomenological modeling: method assessment and formulation suitability. J Pharm Sci. 2002;91(3):631–9.
Feng T, Wang F, Pinal R, Wassgren C, Carvajal MT. Investigation of the variability of NIR in-line monitoring of roller compaction process by using Fast Fourier Transform (FFT) analysis. Aaps Pharmscitech. 2008;9(2):419–24.
Koller DM, Posch A, Horl G, Voura C, Radl S, Urbanetz N, et al. Continuous quantitative monitoring of powder mixing dynamics by near-infrared spectroscopy. Powder Technol. 2011;205(1-3):87–96.
Tumuluri SVS, Prodduturi S, Crowley MM, Stodghill SP, McGinity JW, Repka MA. The use of near-infrared spectroscopy for the quantitation of a drug in hot-melt extruded films. Drug Dev Ind Pharm. 2004;30(5):505–11.
Markovic S, Poljanec K, Kerc J, Horvat M. In-line NIR monitoring of key characteristics of enteric coated pellets. Eur J Pharm Biopharm. 2014;88(3):847–55.
Lyon RC, Lester DS, Lewis EN, Lee E, Yu LX, Jefferson EH, et al. Near-infrared spectral imaging for quality assurance of pharmaceutical products: analysis of tablets to assess powder blend homogeneity. AAPS PharmSciTech. 2002;3(3):1–15.
Sulub Y, Konigsberger M, Cheney J. Blend uniformity end-point determination using near-infrared spectroscopy and multivariate calibration. J Pharm Biomed Anal. 2011;55(3):429–34.
Rantanen J, Jørgensen A, Räsänen E, Luukkonen P, Airaksinen S, Raiman J. Process analysis of fluidized bed granulation. AAPS PharmSciTech. 2001;2(4):21.
Roggo Y, Chalus P, Maurer L, Lema-Martinez C, Edmond A, Jent N. A review of near infrared spectroscopy and chemometrics in pharmaceutical technologies. J Pharm Biomed Anal. 2007;44(3 SPEC. ISS):683–700.
Miller CE. Chemometrics in process analytical chemistry. In: Bakeev KA, editor. Process analytical technology spectroscopic tools and implementation strategies for the chemical and pharmaceutical industries. first. Noida: Blackwell Publishing; 2005. p. 226–324.
Lopes JA, Alves TP, Menezes JC. Chemometric Process Analytical Technology (PAT) applications in bioprocess engineering. IFAC Proc Vol IFAC. 2005;38(1):153–8.
Myakalwar AK, Sreedhar S, Barman I, Dingari NC, Venugopal Rao S, Prem Kiran P, et al. Laser-induced breakdown spectroscopy-based investigation and classification of pharmaceutical tablets using multivariate chemometric analysis. Talanta. 2011;87(1):53–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naidu, V.R., Deshpande, R.S., Syed, M.R. et al. PAT-Based Control of Fluid Bed Coating Process Using NIR Spectroscopy to Monitor the Cellulose Coating on Pharmaceutical Pellets. AAPS PharmSciTech 18, 2045–2054 (2017). https://doi.org/10.1208/s12249-016-0680-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0680-2