Abstract
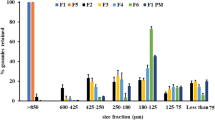
This work aimed at developing enalapril maleate granules in order to improve its stability in solid dosage form. Granules were prepared by hot melt granulation using a fluidized bed apparatus. Gelucire 50/13®, polyethylene glycol 6000 e Poloxamer 407® were studied and compared as binders in 2 × 2 factorial designs where the proportions of enalapril maleate, binders and spray dried lactose were varied. The granulation process resulted in high yields and granule sizes that indicated the prevalence of particles coating. Furthermore, the granules obtained showed adequate flowability and a fast dissolution rate of enalapril maleate with almost 100% of the drug released in 10 min. The stability of enalapril maleate in hard gelatin capsules showed that the drug stability was greatly increased in granules, since for raw drug, the remaining content of enalapril maleate after 91 days was 68.4% and, for granules, the content was always above 93%. This result was confirmed by the quantification of the degradation products, enalaprilat and diketopiperazine, which were found in very low content in granules samples. The results demonstrate that fluidized bed hot melt granulation with hydrophilic binders is a suitable alternative for improving the chemical stability of enalapril maleate.







Similar content being viewed by others
References
Alsante KM, Huynh-Ba K, Baertschi SW, Reed RA, Landis MS, Kleinman MH, et al. Recent trends in product development and regulatory issues on impurities in active pharmaceutical ingredient (API) and drug products. Part 1: predicting degradation related impurities and impurity considerations for pharmaceutical dosage forms. AAPSPharmSciTech. 2014;15:198–212. doi:10.1208/s12249-013-0047-x.
Regulska K, Stanisz B, Lisiecki P. Optimization of storage and manufacture conditions for imidapril hydrochloride in solid state as a way to reduce costs of antihypertensive therapy. AAPSPharmSciTech. 2013;14:1199–208. doi:10.1208/s12249-013-0010-x.
McEvoy GK. AHFS Drug information 1999. 40th Edition Revised American Society of Health-System Pharmacists, February, 1999.
Sosnowska K, Winnicka K, Czajkowska-Koanik A. Stability of extemporaneous enalapril maleate suspensions for pediatric use prepared from commercially available tablets. Acta Pol Pharm - Drug Res. 2009;66:321–6.
Wang SL, Lin SY, Chen TF. Reaction kinetics of solid-state cyclization of enalapril maleate investigated by isothermal FT-IR microscopic system. Chem Pharm Bull. 2001;49:402–6.
Stanisz B. Evaluation of stability of enalapril maleate in solid phase. J Pharm Biomed Anal. 2003;31:375–80.
Diego M, Godoy G, Mennickent S, Godoy R. Chemical stability of enalapril maleate drug substance and tablets by a stability-indicating liquid chromatographic method. Quim Nova. 2011;34:450–4.
Stanisz B. Kinetics of degradation of enalapril maleate in dosage forms. Acta Pol Pharm - Drug Res. 2004;61:415–8.
Al-Omari MM, Abdelah MK, Badwan AA, Jaber AMY. Effect of the drug-matrix on the stability of enalapril maleate in tablet formulations. J Pharm Biomed Anal. 2001;25:893–902.
Lima DM, Santos LD, Lima EM. Stability and in vitro release profile of enalapril maleate from different commercially available tablets: possible therapeutic implications. J Pharm Biomed Anal. 2008;47:934–7.
Resende RLO, Santoro MIRM, Matos JR. Stability and compatibility study on enalapril maleate using thermoanalytical techniques. J Therm Anal Calorim. 2008;93:881–6.
Cotton ML, Wu DW, Vadas EB. Drug-excipient interaction study of enalapril maleate using thermal analysis and scanning electron microscopy. Int J Pharm. 1987;40:129–42.
Merslavic SM, Jozica Razen NM, Rotar LR. Stable formulation of enalapril salt, a process for the preparation thereof and the use thereof. WO Patent. 1994.
Chen J, Zhang LH, Xu RJ, Bu NJ, Zhang L. Proposal of a new degradation mechanism of enalapril maleate and improvement of enalapril maleate stability in tablet formulation with different stabilizers. Pharmazie. 2014;69:277–80.
Cunha TA, Serpa RC, Oliveira APM, Nasser LN, Freitas LAP, Taveira SF, et al. Effect of stearic acid on enalapril stability and dissolution from multiparticulate solid dosage forms. AAPSPharmSciTech. 2013;14:1150–7.
Oliveira APM, Cunha TA, Serpa RC, Taveira SF, Lima EM, Freitas LAP, et al. Improvement of enalapril maleate chemical stability by high shear melting granulation. Pharm Dev Technol. 2014;20(08):1002–8. doi:10.3109/10837450.2014.959178.
Zoppi A, Garnero C, Linck YG, Chattah AK, Monti GA, Longhi MR. Enalapril: β-CD complex: stability enhancement in solid state. Carbohydr Polym. 2011;86:716–21.
Thomsen R, Rasmussen HB, Linnet K. In vitro drug metabolism by human carboxylesterase 1: focus on angiotensin-converting enzyme inhibitors. Drug Metab Dispos. 2014;42:126–33.
Borini GB, Andrade TC, Freitas LAP. Hot melt granulation of coarse pharmaceutical powders in a spouted bed. Powder Technol. 2009;189:520–7.
Rantanen J, Jørgensen A, Räsänen E, Luukkonen P, Airaksinen S, Raiman J, et al. Process analysis of fluidized bed granulation. AAPSPharmSciTech. 2001;2:13–20. doi:10.1007/BF02830561.
Hu X, Li Y, Zhang E, Wang X, Xing M, Wang Q, et al. Preparation and evaluation of orally disintegrating tablets containing taste-masked microcapsules of berberine hydrochloride. AAPSPharmSciTech. 2013;14:29–37.
Silva CAM, Taranto OM. Real-time monitoring of gas–solid fluidized-bed granulation and coating process: evolution of particle size, fluidization regime transitions, and psychometric parameters. Drying Technol. 2015;33:1929–48. doi:10.1080/07373937.2015.1076000.
Andrade TC, Martins RM, Freitas LAP. Granulation of indomethacin and a hydrophilic carrier by fluidized hot melt method: the drug solubility enhancement. Powder Technol. 2015;270:453–60.
Kowalski J, Kalb O, Joshi YM, Serajuddin ATM. Application of melt granulation technology to enhance stability of a moisture sensitive immediate-release drug product. Int J Pharm. 2009;381:56–61.
Walker GM, Holland CR, Ahmad MMN, Craig DQM. Influence of process parameters on fluidized hot melt granulation and tablet pressing of pharmaceutical powders. Chem Eng Sci. 2005;60:3867–77.
Kotyian PN, Yang D, Kulkarini R. Effect of the melt granulation technique on the dissolution characteristics of griseofulvin. Int J Pharm. 2007;329:72–80.
Jivraj M, Martini LG, Thomson CM. An overview of the different excipients useful for the direct compression of tablets. Pharm Sci Technol Today. 2000;3:58–63.
Kidokoro M, Haramiishi Y, Sagasaki S, Shimizu T, Yamamoto Y. Application of fluidized hot-melt granulation for the preparation of granules for tableting; properties of granules and tablets prepared by fluidized hot-melt granulation. Drug Dev Ind Pharm. 2002;28:67–76.
Sharma VD, Akocak S, Ilies MA, Fassihi R. Solid-state interactions at the core-coat interface: physicochemical characterization of enteric-coated omeprazole pellets without a protective sub-coat. AAPSPharmSciTech. 2015;16:934–43. doi:10.1208/s12249-014-0263-z.
Morin G, Briens L. A comparison of granules produced by high-shear and fluidized-bed granulation methods. AAPSPharmSciTech. 2014;15:1039–48. doi:10.1208/s12249-014-0134-7.
Yamamoto K, Shao ZJ. Process development and scale-up: fluid-bed granulation. Chap 30. In: Qiu Y, Chen Y, Zhang GGZ, editors. Developing solid oral dosage forms. New York: Academic Press (Elsevier); 2009. p. 701–14.
Box GEP, Hunter JS, Hunter WG. Statistics for experimenters: design, innovation and discovery, 2nd edition, Wiley-Interscience, 2005, 633p.
Convention USP. United States Pharmacopeia XXXI. National Formulary, United States Pharmacopeia Convention, Rockville, 2008.
International Conference on Harmonization Guideline Q8(2) Pharmaceutical development. November 2008. (Accessed 8th Jan 2015). http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM073507.pdf.
Wells JI. Pharmaceutical preformulation: the physicochemical properties of drug substances. West Sussex: Ellis Horwood Ltd. Publ; 1988. p. 227.
Shah RB, Tawakkul M, Khan MA. Comparative evaluation of flow for pharmaceutical powders and granules. AAPSPharmSciTech. 2008;9:250–8. doi:10.1208/s12249-008-9046-8.
Amidon GE, Secreast PJ, Mudie D. Particle, powder and compact characterization. Chap 8. In: Qiu Y, Chen Y, Zhang GGZ, editors. Developing solid oral dosage forms. New York: Academic Press (Elsevier); 2009. p. 163–86.
Long M, Chen Y. Dissolution testing of solid products. Chap 14. In: Qiu Y, Chen Y, Zhang GGZ, editors. Developing solid oral dosage forms. New York: Academic Press (Elsevier); 2009. p. 319–40.
Zhou D, Porter WR, Zhang GGZ. Drug stability and degradation studies. Chap 5. In: Qiu Y, Chen Y, Zhang GGZ, editors. Developing solid oral dosage forms. New York: Academic Press (Elsevier); 2009. p. 87–124.
Ip DP, Brenner GS, Stevenson JM, Lindenbaum S, Douglas AW, Klein SD, et al. High resolution spectroscopic evidence and solution calorimetry studies on the polymorphs of enalapril maleate. Int J Pharm. 1986;28:183–91.
Pandey P, Hamey R, Bindra DS, Huang Z, Mathias N, Eley T, et al. From bench to humans: formulation development of poorly water soluble drug to mitigate food effect. AAPSPharmSciTech. 2014;15:407–16. doi:10.1208/S12249-013-0069-4.
Sundeep S. Dhareshwar. Case study: enalapril: a prodrug of enalaprilat. In: Prodrugs, Chap 5. Springer Int Publ AG. pp 1221–1229. 2007. doi:10.1007/978-0-387-49785-3-39.
Khawam A, Flanagan DR. Solid-state kinetic models: basics and mathematical fundamentals. J Phys Chem B. 2006;110:17315–28. doi:10.1021/jp062746a.
Khawam A, Flanagam DR. Basics and applications of solid-state kinetics: a pharmaceutical perspective. J Pharm Sci. 2006;95:472–98.
ACKNOWLEDGMENTS
The financial support from FAPESP (2005/05191-2 and 2011/20872-7), CNPq (PQ-2), CAPES (PhD scholarship to T.F. Guimarães) and FAPEG is gratefully acknowledged. Authors would like to thank Rudolf Barth Electron Microscopy Platform of the Oswaldo Cruz Institute/FIOCRUZ for the SEM operation and micrographs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guimarães, T.F., Comelli, A.C.C., Tacón, L.A. et al. Fluidized Bed Hot Melt Granulation with Hydrophilic Materials Improves Enalapril Maleate Stability. AAPS PharmSciTech 18, 1302–1310 (2017). https://doi.org/10.1208/s12249-016-0593-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0593-0