Abstract
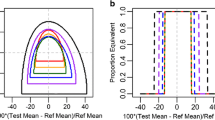
The goal of this article is to discuss considerations regarding implementation of the parametric tolerance interval two one-sided test (PTI-TOST) for delivered dose uniformity (DDU) of orally inhaled products (OIPs). That test was proposed by FDA in 2005 as an alternative to the counting test described in the 1998 draft FDA guidance for metered dose inhalers and dry powder inhalers. The 2005 PTI-TOST, however, still has not found much use in practice despite the general desirability of parametric approaches in modern pharmaceutical quality control. A key reason for its slow uptake is that it rejects, with high probability, batches whose quality is considered acceptable by all other published regulatory and pharmacopeial standards as well as by the DDU specifications for many approved OIPs. Manufacturers therefore continue using nonparametric counting tests for control of DDU. A simulated case study presented here compares the consequences of the PTI-TOST compared to the counting test. The article discusses three possibilities that would help increase the uptake of the PTI-TOST approach, namely: product-specific quality standards, a different default standard suitable for the majority of OIPs, and integration of the PTI-TOST with a continuous verification control strategy rather than using it as an isolated-batch (transactional) end-product testing. In any of these efforts, if a parametric test is used, it is critical not to set the target quality close to, or at the boundary of the process/product capabilities, because PTI tests are designed to reject with high probability the identified target quality.




Similar content being viewed by others
REFERENCES
U.S. Pharmacopeia. USP 34 Chapter <601>.
FDA/CDER. Draft Guidance for Industry. Metered Dose Inhaler (MDI) and Dry Powder Inhaler (DPI) Drug Products Chemistry, Manufacturing, and Controls Documentation 1998. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070573.pdf Accessed May 7, 2010.
Murphy JR and Griffiths KL. Zero-Tolerance Criteria Do Not Assure Product Quality. Pharm Technol. 2006;30(1):52–60. http://www.pharmtech.com/pharmtech/article/articleDetail.jsp?id=283486 Accessed May 8, 2010.
Nasr MM. Paramatric Tolerance Interval Test for Delivered Dose Uniformity. Presentation to the FDA Advisory Committee. 2005. http://www.fda.gov/ohrms/dockets/ac/05/slides/2005-4187S1_13_Nasr.ppt Accessed May 8, 2010.
Golden M. Parametric Tolerance Interval Test for Delivered Dose Uniformity of Orally Inhaled Products. Presentation to the FDA Advisory Committee. 2005. http://www.fda.gov/ohrms/dockets/ac/05/slides/2005-4187S2_10_Golden.ppt Accessed May 8, 2010.
European Pharmacopeia 7.0. Inhalanda Preparations for Inhalations. Monograph 01/2008:0671.
EMEA. Guideline On The Pharmaceutical Quality Of Inhalation And Nasal Products. EMEA/CHMP/QWP/49313/2005. 2006. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003568.pdf Accessed February 8, 2011.
ITFG/IPAC Collaboration, CMC Specifications Technical Team, DCU Working Group. Initial Assessment of the ITFG/IPAC Dose Content Uniformity Database by the CMC Specifications Technical Team of the ITFG/IPAC Collaboration. 2000. http://www.fda.gov/ohrms/dockets/ac/00/techrepro/3609_rpt1.pdf and http://ipacrs.com/PDFs/Initial_Assessment_of_Dose.PDF Accessed February 8, 2011.
Olsson B, Lyapustina S. Debating The Parametric Operating Curves For DDU Tests On Marketed Inhalers. Respiratory Drug Delivery. 2004;1:135–41.
Olsson B. Debating The Operating Curves For DDU Tests On Marketed Inhalers. Presentation at the Respiratory Drug Delivery Conference 2004. Slides 21–24. http://www.ipacrs.com/PDFs/RDD_IX_DDU.PPT Accessed February 8, 2011.
Novick S, Christopher D, Dey M, Lyapustina S, Golden M, Leiner S, et al. A Two One-Sided Parametric Tolerance Interval Test For Control of Delivered Dose Uniformity—Part 1-Characterization of FDA Proposed Test. AAPS PharmSciTech. 2009;10(3):820–8. doi:10.1208/s12249-009-9270-x.
Novick S, Christopher D, Dey M, Lyapustina S, Golden M, Leiner S, et al. A Two One-Sided Parametric Tolerance Interval Test For Control of Delivered Dose Uniformity—Part 2-Effect of Changing Parameters. AAPS PharmSciTech. 2009;10(3):841–9. doi:10.1208/s12249-009-9269-3.
Novick S, Christopher D, Dey M, Lyapustina S, Golden M, Leiner S, et al. A Two One-Sided Parametric Tolerance Interval Test For Control of Delivered Dose Uniformity—Part 3-Investigation of Robustness to Deviations from Normality. AAPS PharmSciTech. 2009;10(3):829–40. doi:10.1208/s12249-009-9271-9.
USP. Chapter <905 > Uniformity of Dosage Units.
FDA. “Drugs@FDA” http://www.accessdata.fda.gov/scripts/cder/drugsatfda/ Accessed April 4, 2011
Tsong Y, Shen M, Lostritto R. Office of Biostatistics, CDER, FDA. Using Two 1-Sided Tolerance Interval Approach To Set Quality Specification A Sampling Acceptance Plan Of Delivery Dose Content Uniformity. Available at http://www.hsph.harvard.edu/ncb2009/files/ncb2009-c07-tsong.ppt See slide 23. Accessed February 8, 2011.
Peri P. Assessing Quality of Inhaled Products And Links to Efficacy and Safety. Presentation at the IPAC-RS March 2011 Conference “Bringing Value to the Patient in a Changing World”. Available at http://www.ipacrs.com/2011%20Conference.html. Accessed April 4, 2011.
ANSI/ASQ Z.19-2008 Sampling Procedures and Tables for Inspection by Variables for Percent Nonconforming.
Olsson B. Debating the Operating Curves for DDU Tests on Marketed Inhalers. Respiratory Drug Delivery. 2004 http://ipacrs.com/PDFs/RDD_IX_DDU.PPT Accessed April 4, 2011.
IPAC-RS. Appendix 1, 1.1 Interpretation of the FDA Tests, in: A Parametric Tolerance Interval Test for Improved Control of Delivered Dose Uniformity of Orally Inhaled and Nasal Drug Products. Submitted to FDA. 2001; 47. http://ipacrs.com/PDFs/IPAC-RS_DDU_Proposal.PDF Accessed July 28, 2011.
Larner G, on behalf of the IPAC-RS DDU/PTIT Working Group. Delivered Dose Uniformity and Parametric Tolerance Interval Testing of OINDPs. 2011. http://www.ipacrs.com/2011%20Conference.html Accessed February 8, 2011.
Davis B, Lundsberg L, Cook G. PQLI Control Strategy Model and Concepts. J Pharm Innov (2008) 3:95–104. doi:10.1007/s12247-008-9035-1. http://www.springerlink.com/content/8t77110318773767/fulltext.pdf Accessed April 4, 2011.
FDA/CDER. Guidance for Industry. Q8(R2) Pharmaceutical Development. 2009. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM073507.pdf. Accessed April 1, 2011.
Strickland H, Clewley L. How PTI-TOST, Control Strategy Principles and Acceptance Sampling Consensus Standards can Work Together to Achieve an Appropriate Quality Assessment Strategy of Delivered Dose Uniformity of OIPs. Poster at the IPAC-RS 2011 Conference. http://ipacrs.com/PDFs/IPAC-RS%202011%20Conference/Posters/16-DDU.pdf or http://ipacrs.com/posters2011.html or http://ipacrs.com/2011%20Conference.html Accessed April 14, 2011.
ACKNOWLEDGEMENTS
The authors thank Monisha Dey for help with the SAS code for the operating characteristic curves, the IPAC-RS DDU Working Group members for discussion and comments, and the IPAC-RS Board of Directors for support of this project.
Author information
Authors and Affiliations
Corresponding author
APPENDIX. UNIFORMITY TESTS USED IN COMPARISONS
APPENDIX. UNIFORMITY TESTS USED IN COMPARISONS
For an appropriate comparison of operating characteristic curves, the same sample sizes (10 + 20, in the first and second tier) were used in this article (the complete dose uniformity test in the draft FDA 1998 guidance requires, in addition, a through-container-life test for MDIs and multi-dose DPIs, with the sample size of 9 + 18, which results in further tightening of the overall dose uniformity test for these multi-dose products (2,20)). Each dose is taken from a different inhaler.
Rights and permissions
About this article
Cite this article
Larner, G., Cooper, A., Lyapustina, S. et al. Challenges and Opportunities in Implementing the FDA Default Parametric Tolerance Interval Two One-Sided Test for Delivered Dose Uniformity of Orally Inhaled Products. AAPS PharmSciTech 12, 1144–1156 (2011). https://doi.org/10.1208/s12249-011-9683-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-011-9683-1