Abstract
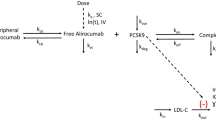
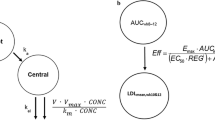
A model-based strategy was used to inform the early clinical development of anacetrapib, a novel cholesteryl ester transfer protein inhibitor under development for the treatment of hyperlipidemia. The objectives of this model-based approach were to enable bridging variable pharmacokinetic effects, differences among formulations used in development, and to identify an appropriate dose for the phase III confirmatory program. Nonlinear mixed effects PK/PD models were initially developed based on data obtained from multiple phase I studies and later were updated with data from a phase IIb study. The population pharmacokinetic model described differences between the liquid-filled capsule used in phase I and phase IIb and the hot-melt extruded (HME) tablet formulation introduced in phase III, allowing for bridging of the two formulations, and quantified the complex relationship of apparent anacetrapib bioavailability with subject meal intake. Proportional E max models quantified the relationships between anacetrapib trough concentration and lipoprotein effects (LDL-C and HDL-C), with covariate effects of study population (normal volunteers vs. patients), and co-administration with HMG-CoA reductase inhibitor (“statin”). The interaction between anacetrapib and atorvastatin suggested pharmacological independence, i.e., that when given together, each agent exerts the same proportional lipid effect observed from monotherapy. Clinical trial simulation was used to examine the robustness of the effects to random dietary indiscretion, and found that the results were robust as long as patients generally adhered to a low-fat diet. These results allowed the selection of the 100 mg dose with the HME formulation for phase III development even though this dose and formulation were not specifically studied in a phase IIb trial.





Similar content being viewed by others
References
Krishna R, Anderson MS, Bergman AJ, Jin B, Fallon M, Cote J, et al. Effect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomized placebo-controlled phase I studies. Lancet. 2007;370:1907–14.
Krishna R, Bergman AJ, Jin B, Fallon M, Cote J, Van Hoydonck P, et al. Multiple-dose pharmacodynamics and pharmacokinetics of anacetrapib, a potent cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjects. Clin Pharmacol Ther. 2008;84(6):679–83.
Bloomfield D, Carlson GL, Sapre A, Tribble D, McKenney JM, Littlejohn 3rd TW, et al. Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patients. Am Heart J. 2009;157(2):352–60.
Krishna R, Garg A, Jin B, Cote J, Bergman A, Von Hoydonck P, et al. Single-dose pharmacokinetics and pharmacodynamics of anacetrapib, a potent cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjects. Br J Clin Pharmacol. 2009;68(4):535–45.
Sheiner LB, Beal SL. NONMEM Users Guide. San Francisco: Division of Pharmacology, University of California; 1979.
Mandema JW, Hermann D, Wang W, Sheiner T, Milad M, Bakker-Arkema R, et al. Model-based development of gemcabene, a novel lipid altering agent. AAPS J. 2005;7(3):E513–22.
Clark RW, Sutfin TA, Suggeri RB, Willauer AT, Sugarman ED, Magnus-Aryitey G, et al. Raising high-density lipoprotein in humans through inhibition of cholestryl ester transfer protein: an initial multidose study of torceterapib. Aterioscler Thromb Vasc Biol. 2004;24:490–7.
Cannon CP, Dansky HM, Davidson M, Gotto Jr AM, Brinton EA, Gould AL, et al. DEFINE investigators. Design of the DEFINE trial: determining the EFficacy and tolerability of CETP INhibition with AnacEtrapib. Am Heart J. 2009;158(4):513–9.
Cannon CP, Shah S, Dansky H, Davidson M, Brinton E, Gotto Jr AM, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med. 2010;363(25):2406–15. doi:10.1056/NEJMoa1009744.
Acknowledgments
The authors like to acknowledge the input of several team members including Alan Hartford and Bo Jin from biostatistics, Daniel Bloomfield from cardiovascular clinical research, Julie Stone and Amit Garg from clinical PK/PD in the model development process and/or interpretation of the results.
Current affiliations: Arthur J. Bergman is presently at Pfizer, Inc., Groton, Connecticut, USA; Marissa F. Dockendorf is at Johnson & Johnson, Inc., Jacksonville, Florida, USA; and Kevin Dykstra is at qPharmetra LLC, Andover, Massachusetts, USA.
Financial Disclosures
This work is sponsored/funded by Merck & Co., Inc. Authors who are employees of Merck & Co., may hold stock or stock options in the company.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: Bernd Meibohm
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. 1
Additional model diagnostic plots for HDL-C exposure/response model. The dashed line is a smooth through the data. The solid line is the line of unity in the upper left hand panel, and a horizontal line with intercept zero in the remaining panels. (TIFF 1,647 kb) (GIF 130 kb)
Fig. 2
Additional model diagnostic plots for LDL-C exposure/response model (TIFF 1,685 kb) (GIF 131 kb)
Fig. 3
Observed and predicted HDL-C versus predicted trough concentration. The patient population includes patients in phase Ib and phase IIb studies treated with and without atorvastatin. The solid line represents the model best fit for the whole population. (TIFF 705 kb) (GIF 42 kb)
Fig. 4
Observed and predicted LDL-C versus trough concentration for the proportional E max model. (TIFF 689 kb) (GIF 38 kb)
Fig. 5
Observed and predicted decrease in LDL-C versus predicted trough concentration for patients treated with anacetrapib or anacetrapib + atorvastatin (proportional E max model). The solid and dashed lines represent the model best fit for the population treated with anacetrapib + atorvastatin and anacetrapib alone, respectively. (TIFF 702 kb) (GIF 41 kb)
Table 1A
Summary of final dataset for population PK model development (DOC 42 kb) (DOC 42 kb)
Table 1B
Summary of final dataset for PD model development (DOC 41 kb) (DOC 41 kb)
Rights and permissions
About this article
Cite this article
Krishna, R., Bergman, A.J., Green, M. et al. Model-Based Development of Anacetrapib, a Novel Cholesteryl Ester Transfer Protein Inhibitor. AAPS J 13, 179–190 (2011). https://doi.org/10.1208/s12248-011-9254-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-011-9254-0