Abstract
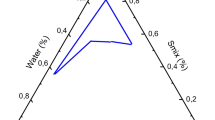
Microemulsions (MEs) are clear, thermodynamically stable systems. They were used to solubilize drugs and to improve topical drug availability. Salicylic acid (SA) is a keratolytic agent used in topical products with antimicrobial actions. The objective of this work was to prepare and evaluate SA ME systems. Different concentrations of SA were incorporated in an ME base composed of isopropyl myristate, water, and Tween 80: propylene glycol in the ratio of 15:1. Three ME systems were prepared: S2%, S5%, and S10% which contain 2%, 5%, and 10% of SA, respectively. Evaluation by examination under cross-polarizing microscope, measuring of percent transmittance, pH measurement, determination of the specific gravity, assessment of rheological properties, and accelerated stability study were carried out. The data showed that the addition of SA markedly affected the physical properties of the base. All systems were not affected by accelerated stability tests. Stability study for 6 months under ambient conditions was carried out for S10%. No remarkable changes were recorded except a decrease in the viscosity value after 1 month. The results suggested that ME could be a suitable vehicle for topical application of different concentrations of SA.



Similar content being viewed by others
REFERENCES
Collier K, Matalonis S, Owen AJ. Evaluation of permeability enhancement by microemulsion in a caco-2 cell system. Proc Int Symp Control Release Bioact Mater. 1999;26:5444.
Alany RG, Rades T, Agatonovic-Kustrin S, Dvies NM, Tucker IG. Effects of alcohols and diols on the phase behaviour of quaternary systems. Int J Pharm. 2000;196:141–5.
Trotta M, Pattarino F, Grosa G. Formation of lecithin-based microemulsions containing n-alkanol phosphocholines. Int J Pharm. 1998;174:253–9.
Marti-Mestres G, Nielloud F. Emulsions in health care applications—an overview. J Dispers Sci Technol. 2002;23:419–39.
Osborne DW, Ward AJ, O’Neill KJ. Microemulsions as topical drug delivery vehicles: in-vitro transdermal studies of a model hydrophilic drug. J Pharm Pharmacol Comm. 1991;43:451–4.
Jachowicz J, Berthiaume MD. Microemulsions vs. macroemulsions in hair care products. Cosmet Toiletries. 1993;108:65–72.
Orth DS, Widjaja J, Ly L, Cao N, Shapiro WB. Stability and skin persistence of topical products: evaluating the effect of a hydroalcoholic hydroquinone vehicle. Cosmet Toiletries. 1998;113(10):51–63.
Reynolds JEF, editor. Martindale, the extra pharmacopoeia, 30th edition. London: The Pharmaceutical Press; 1993.
Bashir SJ, Dreher F, Chew AL, Zhai H, Levin C, Stern R, et al. Cutaneous bioassay of salicylic acid as a keratolytic. Int J Pharm. 2005;292:187–94.
Leslie KS, Dootson G, Sterling JC. Topical salicylic acid gel as a treatment for molluscum contagiosum in Children. J Derm Treat. 2005;16:336–40.
Park WB, Kim SH, Cho JH, Bang JH, Kim HB, Kim NJ, et al. Effect of salicylic acid on invasion of human vascular endothelial cells by Staphylococcus aureus. FEMS Immunol Med Microbiol. 2007;49:56–61. doi:10.1111/j.1574-695X.2006.00170.
Kupferwasser LI, Yeaman MR, Nast CC, Kupferwasser D, Xiong YQ, Palma M, et al. Salicylic acid attenuates virulence in endovascular infections by targeting global regulatory pathways in Staphylococcus aureus. J Clin Invest. 2003;112(2):222–33. doi:10.1172/JC1200316876.
British Medical Association and the Royal Pharmaceutical Society of Great Britain. British National Formulary, No. 26; 1993. p. 408–10.
Draelos ZD. An evaluation of topical 3% salicylic acid and 1% hydrocortisone in the maintenance of scalp pruritus. J Cosmet Dermatol. 2005;4:193–7.
Lee HS, Kim IH. Salicylic acid peels for the treatment of acne vulgaris in Asian patients. Dermatol Surg. 2003;29:1196–9.
Pearl E, Grimes MD. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg. 1999;25(1):18–22.
Ahn HH, Kim IH. Whitening effect of salicylic acid peels in Asian patients. Dermatol Surg. 2006;32:372–5. doi:10.1111/j.1524-4725.2006.32075.
Gladstone HB, Nguyen BA, Williams R, Ottomeyer T, Wortzman M, Jeffers M, et al. Efficacy of hydroquinone cream (USP 4%) used alone or in combination with salicylic acid peels in improving photodamage on the neck and upper chest. Dermatol Surg. 2000;26(4):333–7.
Dainichi T, Ueda S, Isoda M, Koga T, Kinukawa N, Nose Y, et al. Chemical peeling with salicylic acid in polyethylene glycol vehicle suppresses skin tumour development in hairless mice. Br J Dermatol. 2003;148:906–12.
Klimowicz A, Farfał S, Bielecka-Grzela S. Evaluation of skin penetration of topically applied drugs in humans by cutaneous microdialysis: acyclovir vs. salicylic acid. J Clin Pharm Ther. 2007;32:143–8.
Badawi AA, Nour SA, Sakran WS, El-Mancy SS. Formulation and evaluation of new emulsion systems as dosage forms. MD thesis submitted to faculty of pharmacy, Egypt: Cairo University; 2006.
Malcolmson C, Lawrence M. Three-component non-ionic oil-in-water microemulsions using polyoxyethylene ether surfactants. Colloids Surf., B Biointerfaces. 1995;4:97–109.
Constantinides PP, Scalart JP, Lancaster C, Marcello J, Marks G, Ellens H, et al. Formulation and intestinal absorption enhancement evaluation of water-in-oil microemulsions incorporating medium-chain glycerides. Pharm Res. 1994;11(10):1385–90.
Constantinides PP, Welzel G, Ellens H, Smith PL, Sturgis S, Yiv SH, et al. Water-in-oil microemulsions containing medium-chain fatty acids/salts: formulation and intestinal absorption enhancement evaluation. Pharm Res. 1996;13(2):210–5.
Nour SA, Shalaby SH, Afify NN, Abd El-Aal S, Mekhael MK. Formulation and evaluation of econazole nitrate emulgels. J Drug Res Egypt. 2002;24(1–2):63–71.
Lucero MJ, Vigo J, Leon MJ. A study of shear and compression deformations on hydrophilic gels of tretinoin. Int J Pharm. 1994;106:125–33.
Brime B, Moreno MA, Frutos G, Ballesteros MA, Frutos P. Amphotericin B in oil-water lecithin-based microemulsions: formulation and toxicity evaluation. J Pharm Sci. 2002;91(4):1178–85.
Kantaria S, Rees GD, Lawrence MJ. Gelatin-stabilized microemulsion-based organogels: rheology and application in iontophoretic transdermal drug delivery. J Control Release. 1999;60:355–65.
Baudonnet L, Grossiord J-L, Rodriguez F. Physicochemical characterization and in vitro release of salicylic acid from o/w emulsions prepared with Montanov 68®: effect of formulation parameters. Drug Dev Ind Pharm. 2004;30(11):975–84.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Badawi, A.A., Nour, S.A., Sakran, W.S. et al. Preparation and Evaluation of Microemulsion Systems Containing Salicylic Acid. AAPS PharmSciTech 10, 1081–1084 (2009). https://doi.org/10.1208/s12249-009-9301-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-009-9301-7