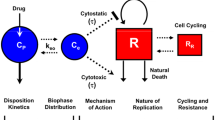
Abstract
Progress in an understanding of the genetic basis of cancer coupled to molecular pharmacology of potential new anticancer drugs calls for new approaches that are able to address key issues in the drug development process, including pharmacokinetic (PK) and pharmacodynamic (PD) relationships. The incorporation of predictive preclinical PK/PD models into rationally designed early-stage clinical trials offers a promising way to relieve a significant bottleneck in the drug discovery pipeline. The aim of the current review is to discuss some considerations for how quantitative PK and PD analyses for anticancer drugs may be conducted and integrated into a global translational effort, and the importance of examining drug disposition and dynamics in target tissues to support the development of preclinical PK/PD models that can be subsequently extrapolated to predict pharmacologic characteristics in patients. In this article, we describe three different physiologically based (PB) PK modeling approaches, i.e., the whole-body PBPK model, the hybrid PBPK model, and the two-pore model for macromolecules, as well as their applications. General conclusions are that greater effort should be made to generate more clinical data that could validate scaled preclinical PB-PK/PD tumor-based models and, thus, stimulate a framework for preclinical to clinical translation. Finally, given the innovative techniques to measure tissue drug concentrations and associated biomarkers of drug responses, development of predictive PK/PD models will become a standard approach for drug discovery and development.



Similar content being viewed by others
References
Hanahan D, Weinberg RA. Hallmarks of cancer. Cell. 2000;100:57–70.
Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719–24.
Collins I, Workman P. New approaches to molecular cancer therapeutics. Nat Chem Biol. 2006;2:689–700.
van Montfort R, Workman P. Structure-based design of molecular cancer therapeutics. Trends Biotechnol. 2009;27:315–28.
Tothfalusi L, Speidl S, Endrenyi L. Exposure-response analysis reveals that clinically important toxicity difference can exist between bioequivalent carbamazepine tablets. Br J Clin Pharmacol. 2008;65:110–22.
Kimko HC, Reele SS, Holford NH, Peck CC. Prediction of the outcome of a phase 3 clinical trial of an antischizophrenic agent (quetiapine fumarate) by simulation with a population pharmacokinetic and pharmacodynamic model. Clin Pharmacol Ther. 2000;68:568–77.
Zhou Q, Guo P, Kruh GD, Vicini P, Wang X, Gallo JM. Predicting human tumor drug concentrations from a preclinical pharmacokinetic model of temozolomide brain disposition. Clin Cancer Res. 2007;13:4271–9.
Wang S, Zhou Q, Gallo JM. Demonstration of the equivalent pharmacokinetic/pharmacodynamic dosing strategy in a multiple-dose study of gefitinib. Mol Cancer Ther. 2009;8:1438–47.
Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York: Marcel Dekker; 1982.
Rowland M, Balant L, Peck C. AAPS J. Physiologically based pharmacokinetics in Drug Development and Regulatory Science: a workshop report (Georgetown University, Washington, DC, May 29–30, 2002). 2004;6:56–67.
Gerlowski LE, Jain RK. Physiologically based pharmacokinetic modeling: principles and applications. J Pharm Sci. 1983;72:1103–27.
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94:1259–76.
Lavé T, Parrott N, Grimm HP, Fleury A, Reddy M. Challenges and opportunities with modelling and simulation in drug discovery and drug development. Xenobiotica. 2007;37:1295–310.
Rocchetti M, Del Bene F, Germani M, Fiorentini F, Poggesi I, Pesenti E, et al. Testing additivity of anticancer agents in pre-clinical studies: a PK/PD modelling approach. Eur J Cancer. 2009;45:3336–46.
Takimoto CH. Pharmacokinetics and pharmacodynamic biomarkers in early oncology drug development. Eur J Cancer. 2009;45 Suppl 1:436–8.
Yap TA, Sandhu SK, Workman P, de Bono JS. Envisioning the future of early anticancer drug development. Nat Rev Cancer. 2010;10:514–23.
Kristjansen PE, Brown TJ, Shipley LA, Jain RK. Intratumor pharmacokinetics, flow resistance, and metabolism during gemcitabine infusion in ex vivo perfused human small cell lung cancer. Clin Cancer Res. 1996;2:359–67.
Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–13.
Ruenraroengsak P, Cook JM, Florence AT. Nanosystem drug targeting: facing up to complex realities. J Control Release. 2010;141:265–76.
Laplanche R, Meno-Tetang GM, Kawai R. Physiologically based pharmacokinetic (PBPK) modeling of everolimus (RAD001) in rats involving non-linear tissue uptake. J Pharmacokinet Pharmacodyn. 2007;34:373–400.
Zager MG, Schlosser PM, Tran HT. A delayed nonlinear PBPK model for genistein dosimetry in rats. Bull Math Biol. 2007;69(1):93–117.
Bradshaw-Pierce EL, Eckhardt SG, Gustafson DL. A physiologically based pharmacokinetic model of docetaxel disposition: from mouse to man. Clin Cancer Res. 2007;13:2768–76.
Shah DK, Shin BS, Veith J, Tóth K, Bernacki RJ, Balthasar JP. Use of an anti-vascular endothelial growth factor antibody in a pharmacokinetic strategy to increase the efficacy of intraperitoneal chemotherapy. J Pharmacol Exp Ther. 2009;329:580–91.
Li M, Al-Jamal KT, Kostarelos K, Reineke J. Physiologically based pharmacokinetic modeling of nanoparticles. ACS Nano. 2010;4:6303–17.
Xu L, Eiseman JL, Egorin MJ, D'Argenio DZ. Physiologically-based pharmacokinetics and molecular pharmacodynamics of 17-(allylamino)-17-demethoxygeldanamycin and its active metabolite in tumor-bearing mice. J Pharmacokinet Pharmacodyn. 2003;30:185–219.
Gupta N, Saleem A, Kötz B, Osman S, Aboagye EO, Phillips R, et al. Carbogen and nicotinamide increase blood flow and 5-fluorouracil delivery but not 5-fluorouracil retention in colorectal cancer metastases in patients. Clin Cancer Res. 2006;12:3115–23.
Saleem A, Price PM. Early tumor drug pharmacokinetics is influenced by tumor perfusion but not plasma drug exposure. Clin Cancer Res. 2008;14:8184–90.
Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man. Theory, procedure and normal values. J Clin Invest. 1948;27:476–83.
Poulin P, Theil FP. Prediction of pharmacokinetics prior to in vivo studies. II. Generic physiologically based pharmacokinetic models of drug disposition. J Pharm Sci. 2002;91:1358–70.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95:1238–57.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–5.
Silva AC, Kim SG, Garwood M. Imaging blood flow in brain tumors using arterial spin labeling. Magn Reson Med. 2000;44:169–73.
Dedrick RL, Zaharko DS, Bender RA, Bleyer WA, Lutz RJ. Pharmacokinetic considerations on resistance to anticancer drugs. Cancer Chemother Rep. 1975;59:795–804.
Lutz RJ, Dedrick RL, Straw JA, Hart MM, Klubes P, Zaharko DS. The kinetics of methotrexate distribution in spontaneous canine lymphosarcoma. J Pharmacokinet Biopharm. 1975;3:77–97.
Weissbrod JM, Jain RK, Sirotnak FM. Pharmacokinetics of methotrexate in leukemia cells: effect of dose and mode of injection. J Pharmacokinet Biopharm. 1978;6:487–503.
Gallo JM, Etse JT, Doshi KJ, Boudinot FD, Chu CK. Hybrid pharmacokinetic models to describe anti-HIV nucleoside brain disposition following parent and prodrug administration in mice. Pharm Res. 1991;8:247–53.
Devineni D, Klein-Szanto A, Gallo JM. In vivo microdialysis to characterize drug transport in brain tumors: analysis of methotrexate uptake in rat glioma-2 (RG-2)-bearing rats. Cancer Chemother Pharmacol. 1996;38:499–507.
Gallo JM, Vicini P, Orlansky A, Li S, Zhou F, Ma J, et al. Pharmacokinetic model-predicted anticancer drug concentrations in human tumors. Clin Cancer Res. 2004;10:8048–58.
Wang S, Guo P, Wang X, Zhou Q, Gallo JM. Preclinical pharmacokinetic/pharmacodynamic models of gefitinib and the design of equivalent dosing regimens in EGFR wild-type and mutant tumor models. Mol Cancer Ther. 2008;7:407–17.
Ostermann S, Csajka C, Buclin T, Leyvraz S, Lejeune F, Decosterd LA, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10:3728–36.
Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res. 2009;15:7092–8.
Lu JF, Eppler SM, Wolf J, Hamilton M, Rakhit A, Bruno R, et al. Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clin Pharmacol Ther. 2006;80:136–45.
Joerger M, Huitema AD, Huizing MT, Willemse PH, de Graeff A, Rosing H, et al. Safety and pharmacology of paclitaxel in patients with impaired liver function: a population pharmacokinetic-pharmacodynamic study. Br J Clin Pharmacol. 2007;64:622–33.
Zandvliet AS, Schellens JH, Dittrich C, Wanders J, Beijnen JH, Huitema AD. Population pharmacokinetic and pharmacodynamic analysis to support treatment optimization of combination chemotherapy with indisulam and carboplatin. Br J Clin Pharmacol. 2008;66:485–97.
Fetterly GJ, Grasela TH, Sherman JW, Dul JL, Grahn A, Lecomte D, et al. Pharmacokinetic/pharmacodynamic modeling and simulation of neutropenia during phase I development of liposome-entrapped paclitaxel. Clin Cancer Res. 2008;14:5856–63.
La Rosée P, O'Dwyer ME, Druker BJ. Insights from pre-clinical studies for new combination treatment regimens with the Bcr-Abl kinase inhibitor imatinib mesylate (Gleevec/Glivec) in chronic myelogenous leukemia: a translational perspective. Leukemia. 2002;16:1213–9.
Mager DE, Wyska E, Jusko WJ. Diversity of mechanism-based pharmacodynamic models. Drug Metab Dispos. 2003;31:510–8.
Jusko WJ, Ko HC. Physiologic indirect response models characterize diverse types of pharmacodynamic effects. Clin Pharmacol Ther. 1994;56:406–19.
Sheiner LB, Verotta D. Further notes on physiologic indirect response models. Clin Pharmacol Ther. 1995;58:238–40.
Luo FR, Yang Z, Dong H, Camuso A, McGlinchey K, Fager K, et al. Prediction of active drug plasma concentrations achieved in cancer patients by pharmacodynamic biomarkers identified from the geo human colon carcinoma xenograft model. Clin Cancer Res. 2005;11:5558–65.
Sung JH, Dhiman A, Shuler ML. A combined pharmacokinetic-pharmacodynamic (PK-PD) model for tumor growth in the rat with UFT administration. J Pharm Sci. 2009;98:1885–904.
Koch G, Walz A, Lahu G, Schropp J. Modeling of tumor growth and anticancer effects of combination therapy. J Pharmacokinet Pharmacodyn. 2009;36:179–97.
Dagnino G, Donelli MG, Colombo T, Bertello C, Pacciarini MA, Martini A. Pharmacodynamic model describing the growth of a mammary carcinoma in the mouse under the influence of adriamycin treatment. Oncology. 1981;38:53–8.
Bueno L, de Alwis DP, Pitou C, Yingling J, Lahn M, Glatt S, et al. Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur J Cancer. 2008;44:142–50.
Tham LS, Wang L, Soo RA, Lee SC, Lee HS, Yong WP, et al. A pharmacodynamic model for the time course of tumor shrinkage by gemcitabine+carboplatin in non-small cell lung cancer patients. Clin Cancer Res. 2008;14:4213–8.
Salphati L, Wong H, Belvin M, Bradford D, Edgar KA, Prior WW, et al. Pharmacokinetic-pharmacodynamic modeling of tumor growth inhibition and biomarker modulation by the novel PI3K inhibitor 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941). Drug Metab Dispos. 2010;38:1436–42.
Soto E, Staab A, Freiwald M, Munzert G, Fritsch H, Döge C, et al. Prediction of neutropenia-related effects of a new combination therapy with the anticancer drugs BI 2536 (a Plk1 inhibitor) and pemetrexed. Clin Pharmacol Ther. 2010;88:660–7.
Baxter LT, Zhu H, Mackensen DG, Jain RK. Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res. 1994;54:1517–28.
Baxter LT, Zhu H, Mackensen DG, Butler WF, Jain RK. Biodistribution of monoclonal antibodies: scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res. 1995;55:4611–22.
Ferl GZ, Kenanova V, Wu AM, DiStefano 3rd JJ. A two-tiered physiologically based model for dually labeled single-chain Fv-Fc antibody fragments. Mol Cancer Ther. 2006;5:1550–8.
Urva SR, Yang VC, Balthasar JP. Physiologically based pharmacokinetic model for T84.66: a monoclonal anti-CEA antibody. J Pharm Sci. 2010;99:1582–600.
Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81–8.
Rippe B, Haraldsson B. Transport of macromolecules across microvascular walls: the two-pore theory. Physiol Rev. 1994;74:163–219.
Himmelstein KJ, Lutz RJ. A review of the applications of physiologically based pharmacokinetic modeling. J Pharmacokinet Biopharm. 1979;7:127–45.
Mordenti J. Pharmacokinetic scale-up: accurate prediction of human pharmacokinetic profiles from animal data. J Pharm Sci. 1985;74:1097–9.
Tsuji A, Nishide K, Minami H, Nakashima E, Terasaki T, Yamana T. Physiologically based pharmacokinetic model for cefazolin in rabbits and its preliminary extrapolation to man. Drug Metab Dispos. 1985;13:729–39.
Hosseini-Yeganeh M, McLachlan AJ. Physiologically based pharmacokinetic model for terbinafine in rats and humans. Antimicrob Agents Chemother. 2002;46:2219–28.
Brightman FA, Leahy DE, Searle GE, Thomas S. Application of a generic physiologically based pharmacokinetic model to the estimation of xenobiotic levels in human plasma. Drug Metab Dispos. 2006;34:94–101.
Mager DE, Woo S, Jusko WJ. Scaling pharmacodynamics from in vitro and preclinical animal studies to humans. Drug Metab Pharmacokinet. 2009;24:16–24.
Kagan L, Abraham AK, Harrold JM, Mager DE. Interspecies scaling of receptor-mediated pharmacokinetics and pharmacodynamics of type I interferons. Pharm Res. 2010;27:920–32.
Acknowledgments
Research by the authors is supported by NIH grants CA127963, CA127963-S1, and CA072937.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Robert M. Straubinger and Donald E. Mager
Rights and permissions
About this article
Cite this article
Zhou, Q., Gallo, J.M. The Pharmacokinetic/Pharmacodynamic Pipeline: Translating Anticancer Drug Pharmacology to the Clinic. AAPS J 13, 111–120 (2011). https://doi.org/10.1208/s12248-011-9253-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-011-9253-1