Abstract
Plasmacytoid (PUC) variant is a rare and aggressive form of urothelial cancer representing 1 to 3% of the bladder cancer. The main differential diagnosis is the bladder involvement by lymphoma-plasmocytoma or metastasis from lobular breast cancer or diffuse gastric cancer. Immunexpression of cytokeratin 7 and GATA3 is the rule, but CD138 may be positive in high percentage of cases. CDH1 somatic mutation or, more rarely, methylation of the gene promoter is the main genetic characteristic of PUC, but germinative mutation is always negative. The recognition of this special histology is very important for the correct management of the patients because of the high rate of positive surgical margins and atypical disease progression. PUC is responsive to cisplatin-based chemotherapy but recurrence is the rule. Peritoneal dissemination is frequent and cancer specific mortality is as high as 56% in a range of 19 to 23 months.
Similar content being viewed by others
Introduction
Bladder cancer is the 10th most common form of cancer worldwide, with an estimated 549,000 new cases and 200,000 deaths in 2018 (Bray et al. 2018).
The WHO publication of 2016 recognizes 10 variants of urothelial carcinoma (UC), significant from the diagnostic, prognostic, and/or therapeutic perspective (Table 1).
In 1991 Sahin et al. (Sahin et al. 1991) and Zukerberg et al. (Zukerberg et al. 1991) described almost simultaneously a new variant of bladder cancer simulating lymphoma, that was later recognized by the World Health Organization (WHO) classification system in 2004. This rare and very aggressive form is called plasmacytoid urothelial carcinoma (PUC), also known as poorly cohesive or diffuse carcinoma.
This review will describe the clinical, histological, immunohistochemical and molecular aspects of the PUC, whose identification is essential for the correct management of patients.
Epidemiology and clinical features
PUC is a rare variant of bladder cancer, representing 1–3% of urothelial cancer. Eighty to 90% of patients are male and the age of diagnosis ranges from 45 to 89 years old. The main symptoms are gross hematuria, dysuria, nocturia and urinary frequency (Mai et al. 2006; Fritsche et al. 2008; Baldwin et al. 2005; Lopez-Beltran et al. 2009; Fox et al. 2017), although abdominal pain and ascites has been described as a consequence of peritoneal dissemination (Shao et al. 2017; Jibril and Stevens 2018). Unusual presentation as scrotal (Wang et al. 2016) or penile invasion (Messina et al. 2016) and urinary and intestinal obstruction have been reported.
Pathologic findings
There are no details about the gross examination in the literature, but sessile and protruding isolated or multiple tumor masses, as well as diffuse infiltration of the bladder has been described.
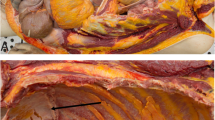
The definition of PUC is variable in the literature, being called plasmacytoid when represents at least 50 to 90% of the tumor, but others consider any percentage suitable for this classification (Li et al. 2019). PUC are by definition a high-grade urothelial cancer. Tumor cells are small to medium size, discohesive with eccentrically placed oval to round, and hyperchromatic nuclei. The cytoplasm is moderate to abundant and eosinophilic, resembling plasma cells. Binucleation is rare and mitotic figures are frequently seen. The nucleoli can be identified but is not prominent in the majority of cases. Plasmacytoid morphology represents between 5 and 100% of the tumor sample (Fig. 1). Around half of them are pure, but conventional UC, sarcomatoid, micropapillary, nested and small cell carcinoma can also be identified. The cells are arranged in cords, single files, small nests, solid sheetlike and occasionally assume a deceptive benign appearance, mimicking an inflammatory process (Fig. 2). The stroma may present a myxoid appearance, and cytoplasm vacuoles can be seen, but true signet cells are not identified (Fig. 3). In 30–43% of the cases vascular invasion is present (Fig. 4). Tumor stage is pT3 or higher in 56–100% and lymph node metastasis is present in 20–73% of the reported cases. Diffuse infiltration pattern, local spread and extension along pelvic fascial planes, involving perivesical, perirectal, and periureteric soft tissues are very common (Fig. 5) (Kaimakliotis et al. 2014a), and the peritoneal spread, occurs in 33–68% of patients (Sato et al. 2009; Ricardo-Gonzalez et al. 2012). Because of these characteristics, it is critical for pathologists to recognize PUC preoperatively, for prognostic and therapeutic purposes, including orientation regarding surgical margins. The rate of positive radical surgical margin ranges from 11 to 60%, and ureteral margin can be positive in up to one third of the cases, which is much more then <4% of conventional UC (Kaimakliotis et al. 2014a; Cockerill et al. 2017).
The immunohistochemical profile (Fig. 6) shows strong and diffuse positivity to CK7 (89–100%) and CK20 (31–100%). CD138 is reported in 11–100%, but LCA is always negative. Considering the differential diagnosis between a primary bladder tumor or spread from the breast or gastrointestinal tract, a panel of 8 markers was proposed by Bohan et al. (Borhan et al. 2017). Gross cystic disease fluid protein 15 (GCDFP-15), progesterone receptors, CDX2, and polyclonal carcinoembryonic antigen (p-CEA) showed positive staining in 24.4, 13.3, 17.7, and 48.8% of the cases, respectively. GATA 3 and uroplakin II immunostaining was expressed in 82.2 and 33.3% cases, respectively. All of the cases of plasmacytoid variant of UC were negative for estrogen receptor (ER) and mammaglobin.
Molecular aspects
All variant bladder cancer histologies were excluded from The Cancer Genome Atlas (TCGA) and their molecular basis remains ill defined. E-Chaderin loss resulting from CDH1 Y68fs mutation is so far typical of PUC, although in rare cases methylation of the gene promoter region has been detected (Al-Ahmadie et al. 2016). E-cadherin encoded by CDH1 Gene is a transmembrane glycoprotein, member of the cadherin family of molecules, predominantly expressed at the basolateral membrane of epithelial cells, where it exerts cell-cell adhesion and invasion-suppression functions (Nagar et al. 1996). It participates in maintenance of polarization and epithelial differentiation during development (Wijnhoven et al. 2000). E-cadherin loss (Fig. 7), leads to the enhanced cellular migration and invasive properties characteristic of plasmacytoid-variant tumors. Study conducted by Al-Ahmadie shows that with the exception of CDH1 alterations the genomic profile of plasmacytoid-variant tumors was not substantially different from the NOS-UC. Frequent mutations in the tumor suppressors TP53 and RB1, in the chromatin remodeler ARID1A, in kinases ERBB2 and PIK3CA and in telomerase reverse transcriptase (TERT) have also be seen in PUC in the TCGA study and in the Memorial Sloan Kettering prospective cohorts (Al-Ahmadie et al. 2016; Palsgrove et al. 2018). Loss of E-cadherin expression by germline mutation seen in most diffuse gastric cancers and in lobular breast cancers (Hirohashi 2000), is not identified in PUC, despite the high morphological similarity with these carcinomas.
Treatment and outcome
Treatment includes surgery, radiotherapy and adjuvant or neoadjuvant chemotherapy, but the optimal treatment strategy has not yet been elucidated due to the small number of patients. Although chemosensitive, recurrence, mainly peritoneal carcinomatosis is common, and survival outcomes are inferior for PUC (Kaimakliotis et al. 2014b; Dayyani et al. 2013a). In the largest series reported, the cancer specific mortality is higher than 56% in a period variable from 19 to 23 months, being the adjusted risk to die from cancer 2.1 for PUC histology compared to NOS-UC (Fox et al. 2017; Dayyani et al. 2013b; Keck et al. 2013). The new gold standard for high grade UC is the neoadjuvant chemotherapy, but the response is variable for different histologies, and there are few reports regarding PUC. Gunaratne et al. (Gunaratne et al. 2016) treated a 58 years-old male with 4 cycles of neoadjuvant gemcitabine and cisplatin that have led to a complete histologic response (pT0). The patient remained free of disease at 14 months of follow-up. On the contrary Dayyani et al. (Dayyani et al. 2013b) treated 5 from 16 patients with localized PUC with neoadjuvant chemotherapy with cisplatin-based regimens (methotrexate/vinblastine/Adriamycin/cisplatin or gemcitabine/cisplatin). There were 4 pathologic downstaging, being 3 complete responses (ypT0N0). Despite the pathological downstaging there was no difference in survival and the peritoneal recurrence was common, even in patients who had pathological complete response. Peritoneal recurrence, infiltration of abdominal wall, scrotum and penile can occur and some response to chemotherapy has been reported (da Fonseca et al. 2014).
Conclusion
Pathologists have to be aware to distinguish and report PUC, an aggressive variant of UC characterized by CDH1 somatic mutation with poor prognosis, locally infiltrative pattern and high risk for relapse despite surgery and chemotherapy.
Availability of data and materials
Review article.
References
Al-Ahmadie HA, Iyer G, Lee BH, Scott SN, Mehra R, Bagrodia A et al (2016) Frequent somatic CDH1 loss-of-function mutations in plasmacytoid variant bladder cancer. Nat Genet 48(4):356–358
Baldwin L, Lee AH, Al-Talib RK, Theaker JM (2005) Transitional cell carcinoma of the bladder mimicking lobular carcinoma of the breast: a discohesive variant of urothelial carcinoma. Histopathology 46(1):50–56
Borhan WM, Cimino-Mathews AM, Montgomery EA, Epstein JI (2017) Immunohistochemical differentiation of Plasmacytoid Urothelial carcinoma from secondary carcinoma involvement of the bladder. Am J Surg Pathol 41(11):1570–1575
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Cockerill PA, Cheville JC, Boorjian SA, Blackburne A, Thapa P, Tarrell RF et al (2017) Outcomes following radical cystectomy for Plasmacytoid Urothelial carcinoma: defining the need for improved local Cancer control. Urology 102:143–147
da Fonseca LG, Souza CE, Mattedi RL, Girardi DM, Sarkis AS, Hoff PMG (2014) Plasmacytoid urothelial carcinoma: a case of histological variant of urinary bladder cancer with aggressive behavior. Autopsy Case Rep 4(4):57–61
Dayyani F, Czerniak BA, Sircar K, Munsell MF, Millikan RE, Dinney CP et al (2013b) Plasmacytoid urothelial carcinoma, a chemosensitive cancer with poor prognosis, and peritoneal carcinomatosis. J Urol 189(5):1656–1661
Dayyani F, Pettaway CA, Kamat AM, Munsell MF, Sircar K, Pagliaro LC (2013a) Retrospective analysis of survival outcomes and the role of cisplatin-based chemotherapy in patients with urethral carcinomas referred to medical oncologists. Urol Oncol 31(7):1171–1177
Fox MD, Xiao L, Zhang M, Kamat AM, Siefker-Radtke A, Zhang L et al (2017) Plasmacytoid Urothelial carcinoma of the urinary bladder: a Clinicopathologic and Immunohistochemical analysis of 49 cases. Am J Clin Pathol 147(5):500–506
Fritsche HM, Burger M, Denzinger S, Legal W, Goebell PJ, Hartmann A (2008) Plasmacytoid urothelial carcinoma of the bladder: histological and clinical features of 5 cases. J Urol 180(5):1923–1927
Gunaratne DA, Krieger LE, Maclean F, Vaux KJ, Chalasani V (2016) Neoadjuvant chemotherapy with gemcitabine and Cisplatin for Plasmacytoid Urothelial bladder Cancer: a case report and review of the literature. Clin Genitourin Cancer 14(1):e103–e105
Hirohashi S (2000) Molecular aspects of adhesion-epigenetic mechanisms for inactivation of the E-cadherin-mediated cell adhesion system in cancers. Verhandlungen der Deutschen Gesellschaft fur Pathologie 84:28–32
Jibril A, Stevens AC (2018) Plasmacytoid Urothelial carcinoma of ureter with retroperitoneal metastasis: a case report. Am J Case Rep 19:158–162
Kaimakliotis HZ, Monn MF, Cary KC, Pedrosa JA, Rice K, Masterson TA et al (2014b) Plasmacytoid variant urothelial bladder cancer: is it time to update the treatment paradigm? Urol Oncol 32(6):833–838
Kaimakliotis HZ, Monn MF, Cheng L, Masterson TA, Cary KC, Pedrosa JA et al (2014a) Plasmacytoid bladder cancer: variant histology with aggressive behavior and a new mode of invasion along fascial planes. Urology 83(5):1112–1116
Keck B, Wach S, Stoehr R, Kunath F, Bertz S, Lehmann J et al (2013) Plasmacytoid variant of bladder cancer defines patients with poor prognosis if treated with cystectomy and adjuvant cisplatin-based chemotherapy. BMC Cancer 13:71
Li Q, Assel M, Benfante NE, Pietzak EJ, Herr HW, Donat M et al (2019) The impact of Plasmacytoid variant histology on the survival of patients with Urothelial carcinoma of bladder after radical cystectomy. Eur Urol Focus 5(1):104–108
Lopez-Beltran A, Requena MJ, Montironi R, Blanca A, Cheng L (2009) Plasmacytoid urothelial carcinoma of the bladder. Hum Pathol 40(7):1023–1028
Mai KT, Park PC, Yazdi HM, Saltel E, Erdogan S, Stinson WA et al (2006) Plasmacytoid urothelial carcinoma of the urinary bladder report of seven new cases. Eur Urol 50(5):1111–1114
Messina C, Zanardi E, Dellepiane C, Tomasello L, Colecchia M, Ravetti GL et al (2016) A case of Plasmacytoid variant of bladder Cancer with a single penile metastasis and a complete response to carboplatin-based chemotherapy and review of the literature. Clin Genitourin Cancer 14(1):e139–e142
Nagar B, Overduin M, Ikura M, Rini JM (1996) Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature 380(6572):360–364
Palsgrove DN, Taheri D, Springer SU, Cowan M, Guner G, Mendoza Rodriguez MA et al (2018) Targeted sequencing of Plasmacytoid urothelial carcinoma reveals frequent TERT promoter mutations. Hum Pathol 85:1–9
Ricardo-Gonzalez RR, Nguyen M, Gokden N, Sangoi AR, Presti JC Jr, McKenney JK (2012) Plasmacytoid carcinoma of the bladder: a urothelial carcinoma variant with a predilection for intraperitoneal spread. J Urol 187(3):852–855
Sahin AA, Myhre M, Ro JY, Sneige N, Dekmezian RH, Ayala AG (1991) Plasmacytoid transitional cell carcinoma. Report of a case with initial presentation mimicking multiple myeloma. Acta Cytol 35(3):277–280
Sato K, Ueda Y, Kawamura K, Aihara K, Katsuda S (2009) Plasmacytoid urothelial carcinoma of the urinary bladder: a case report and immunohistochemical study. Pathol Res Pract 205(3):189–194
Shao YH, Kao CC, Tang SH, Cha TL, Tsao CW, Meng E et al (2017) Unusual presentation of direct intraperitoneal metastases complicated with massive ascites from plasmacytoid variant of bladder cancer and adenocarcinoma of colon: a case report and literature review. Medicine (Baltimore) 96(7):e5816
Wang YG, Perera M, Gleeson J (2016) Plasmacytoid urothelial carcinoma of the bladder with extensive scrotal wall invasion. Urology Annals 8(3):381–383
Wijnhoven BP, Dinjens WN, Pignatelli M (2000) E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg 87(8):992–1005
Zukerberg LR, Harris NL, Young RH (1991) Carcinomas of the urinary bladder simulating malignant lymphoma. A report of five cases. Am J Surg Pathol 15(6):569–576
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
I am the only author. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Review article.
Consent for publication
I am the only author.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Leite, K.R.M. Plasmocytoid urothelial carcinoma - clinical, histological, immunohistochemical and molecular aspects. Surg Exp Pathol 3, 3 (2020). https://doi.org/10.1186/s42047-020-0056-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42047-020-0056-5