Abstract
Background
Malignant phyllodes tumors (PTs) of the breast occur infrequently and are difficult to treat with adjuvant therapy. Here, we present a case of a female patient with a huge malignant PT with rapid progression in a short period.
Case presentation
A 44-year-old woman presented to our hospital with a rapid growth mass in her right breast, measuring 20 cm. She was initially diagnosed as having a borderline phyllodes tumor by core needle biopsy and underwent total mastectomy and artificial dermis was grafted, 20 days later, latissimus dorsi muscle flap and free skin grafting were performed. Two courses of doxorubicin–ifosfamide therapy were administered because of recurrence, but the patient died 4 months after the mastectomy.
Conclusions
A standard therapeutic strategy for malignant PTs is needed in urgently to reduce the risk of tumor recurrence.
Similar content being viewed by others
Background
Phyllodes tumors (PTs) of the breast are extremely rare, globally accounting for 0.3% to 1% of breast tumors [1]. Their name is derived from the Greek phyllon (leaf) because of its lobed histological appearance. It is also known as cystosarcoma phyllodes, adenomatous myxoma, and pseudosarcoma adenoma [1,2,3]. It usually occurs in middle-aged women (age, 35–55 years) [4]. Clinically, the size of the tumor varies between 4 and 7 cm on average [5], but about one-fifth of PTs are called giant PT tumors because of their uncommon diameter of more than 10 cm [6]. Depending on the histopathological features, PTs are categorized into three grades with different proportions, benign (60%–75%), borderline (13%–26%), and malignant (10%–20%) [7]. Patients suffering from PTs have no specific clinical manifestations, and it is difficult to distinguish the benign subgroup from the borderline and malignant subgroups. Malignant PTs are more readily characterized by stromal pleomorphism and overgrowth, frequent mitoses and infiltrative borders [8]. Lymph node metastasis is rare, and the metastatic path relies mainly on the blood. Surgical removal is the primary treatment for PT, given that adjuvant treatments play a poorly efficient role in this malignancy. Here, we report the case of a female patient with a huge malignant PT with rapid progression in a short period.
Case presentation
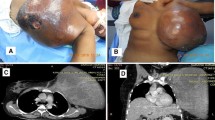
A 44-year-old woman presented to our hospital with a rapid growth mass in her right breast, measuring 20 cm. The breast skin appeared dark with ulceration (Fig. 1). Her family history and past history were unremarkable. One year prior to admission, she incidentally palpated the tumor, and noted gradual growth 2 months prior to the hospital visit. Computed tomography and magnetic resonance imaging revealed a hemorrhagic giant mass with a well-defined border. The axillary and supraclavicular lymph nodes were swollen, but there was no sign of chest wall invasion or distant metastasis (Fig. 2). A diagnosis of PT of borderline malignancy was established on the basis of the core needle biopsy findings. Subsequently, the patient underwent mastectomy of the right breast and axillary lymph node dissection. During surgery, the surgeons detected invasion of the pectoralis major; thus, the partial muscle adhering to the tumor was resected. Because the skin defect was large, artificial dermis [9] was grafted to remedy for the defect (Fig. 3). Twenty days later, latissimus dorsi muscle flap and free skin grafting were performed. The histopathological examination demonstrated that the tumor was composed of atypical spindle-shaped cells with enlarged nuclei and exhibited a fibrosarcoma-like morphology which included ill-defined invasion into the adjacent breast tissue, the overlying skin, and the pectoral muscle (Fig. 4a). Numerous mitoses were noted (10/10 high-power fields) and necrosis had occurred (Fig. 4b). A degenerated leaf-like structure was observed in the center of the lesion (Fig. 4c). Three lymph node metastases with a maximum diameter of 25 mm. were noted. Based on these findings, a final diagnosis of malignant PT was established.
Because 1 month after the mastectomy the tumor had re-grown in the surrounding skin graft and right pleural effusion had appeared (Fig. 5), as an alternative treatment, we administered two courses of doxorubicin–ifosfamide (AI) therapy (30 mg/m2 doxorubicin on days 1–2 and 2 g/m2 ifosfamide on days 1–5) including Mesna (sodium 2-mercaptoethane sulfonate) and sufficient infusion volumes to prevent ifosfamide-related hemorrhagic cystitis. Grade 4 neutropenia and anemia (as defined by the Common Terminology Criteria for Adverse Events) occurred during AI therapy, thus, to prevent the advancement of neutropenia, filgrastim, a granulocyte-colony-stimulating factor, was administered, and transfusion was performed. The local tumor was temporarily reduced after one cycle of AI therapy (Fig. 6); however, after two cycles of AI therapy, the chest wall recurrence and pleural dissemination progressed rapidly (Fig. 7). The patient died 4 months after the mastectomy because of respiratory failure.
Discussion
Malignant PT is rare lesion of the breast that can mimic benign masses such as fibroadenomas, on clinical diagnosis, but is characterized by a typical rapid growth. PTs usually occur in middle-aged women ranging in age from 35 to 55 years, with an average presentation at 45 years [4]. PTs are composed of epithelial elements and a connective tissue stroma with higher stromal cellularity. A malignant PT is distinguished from a benign/borderline PT by the presence of marked stromal cellularity, cellular atypia and mitotic activity in at least 10/10 high-power fields [10].
The clinical presentation and the radiographic findings of malignant PT are strikingly similar to those of benign lesions, such as fibroadenoma, or even benign PT, thus, making it quite challenging for clinicians to diagnose or even to suspect the disease at an early stage. Although routine breast biopsy may not be warranted, it is crucial for clinicians to consider and include PT in their differential diagnosis. Moreover, it is also evident that clinicians cannot rely completely on radiographic findings.
According to the National Comprehensive Cancer Network (NCCN) guidelines for breast cancer, the management of PTs with a size > 3.0 cm is surgical excision with clean margins (≥ 1.0 cm) without axillary staging, regardless of whether the tumor is benign, borderline, or malignant [11]. Many other studies have supported the contention that margins that are ≤ 1.0 cm are associated with a higher recurrence rate, ranging from 16.7 to 40% [4, 12].
The prognosis of PTs is variable, with local recurrence rates ranging from 10 to 40% (average 15%) and distant metastases occurring in 10% of all PTs and up to 20% of malignant PTs [13]. Survival after metastatic disease is poor, with various case series reporting a median survival ranging from 4 to 17 months, with large variability based on the site of the metastatic disease [14]. Other large prospective studies have reported 5-year disease-free survival rates of 96% for benign PTs and 66% for malignant PTs [15]. Most sarcomas metastasize hematogenously, and the incidence of axillary lymph node involvement in malignant PTs ranges from 1.1 to 3.8% [16].
There is currently no consensus regarding the recommendations for radiotherapy, hormonal therapy, and systemic chemotherapy for malignant PTs. To date, no double-blinded, multicenter study has been performed on this subject. Most case reports and studies describe treating these tumors exclusively with wide local excision, in according with the current NCCN guidelines. As PTs are considered as soft-tissue sarcoma, adjuvant chemotherapy with doxorubicin plus dacarbazine may provide some benefits to patients with large (> 5.0 cm), high-risk tumors [17]. A deeper investigation of the addition of adjuvant therapy for large aggressive malignant cases of PT may prove to be fruitful [18]. Recently, doxorubicin and ifosfamide therapy has been reported to be effective to treat metastases of malignant PTs [19, 20]. In accordance with previous reports [19, 20], we administered AI therapy using 60 mg/m2 doxorubicin and 10 g/m2 ifosfamide in each course.
Because there are not many reports of malignant cases of PT, the data available are insufficient to calculate fully the statistics of the survival rate associated with these tumors. Past case reports and studies have suggested that the prognosis of malignant PT of the breast is usually poor, while the overall prognosis of benign PT is good [14, 21].
The early diagnosis and staging of PTs are pivotal not only for improving the overall outcome of the disease after treatment, but also to promote the quality of life of the patient by causing less disfiguration. While the breast cancer screening guideline suggests that women over 40 years of age should begin routine mammograms to detect the presence of breast cancers, PTs can occur a decade before this minimum screening age as they occur during the third or fourth decades of life. Moreover, if the patient suspects that the lesion exhibits growth within 6 months to a year of initial detection, it should be considered for further workup.
Conclusions
In conclusion, malignant PTs are rare entities with distinct clinicopathological features. These tumors should be accurately recognized and effectively treated at first diagnosis, as they have a high risk of recurrence. There is no established consensus regarding the optimal type of surgery and indications for radiotherapy and chemotherapy regimens in these cases. The establishment of standard therapeutic strategy for malignant PTs is needed urgently to reduce the risk of tumor recurrence.
Availability of data and materials
All datasets supporting the conclusions of this article are included within the article.
Abbreviations
- AI:
-
Doxorubicin–ifosfamide
- NCCN:
-
National Comprehensive Cancer Network
- PT:
-
Phyllodes tumor
References
Guerrero MA, Ballard BR, Grau AM. Malignant phyllodes tumor of the breast: review of the literature and case report of stromal overgrowth. Surg Oncol. 2003;12:27–37.
Lakhani SR, Ellis IO, Schnitt SJ. WHO classification of tumours of the breast. 4th ed. Lyon: IARC; 2012. p. 143–7.
Sanguinetti A, Bistoni G, Calzolari F, et al. Cystosarcoma phyllodes with muscular and lymph node metastasis. Our experience and review of the literature. Ann Italy Chir. 2012;83:331–6.
Testori A, Meroni S, Errico V, et al. Huge malignant phyllodes breast tumor: a real entity in a new era of early breast cancer. World J Surg Oncol. 2015;13:81–4.
Barrio AV, Clark BD, Goldberg JI, et al. Clinicopathologic features and long-term outcomes of 293 phyllodes tumors of the breast. Ann Surg Oncol. 2007;14:2961–70.
Mishra SP, Tiwary SK, Mishra M, et al. Phyllodes tumor of breast: a review article. ISRN Surg. 2013;361:469.
Tse GM, Niu Y, Shi HJ. Phyllodes tumor of the breast: an update. Breast Cancer. 2010;17:29–34.
Lee AH. Recent developments in the histological diagnosis of spindle cell carcinoma, fibromatosis and phyllodes tumour of the breast. Histopathology. 2008;52:45–57.
Suzuki S, Kawai K, Ashoori F, et al. Long-term follow-up study of artificial dermis composed of outer silicon layer and inner collagen sponge. Br J Plast Surg. 2000;53:659–66.
Sawyer EJ, Hanby AM, Ellis P, et al. Molecular analysis of phyllodes tumors reveals distinct changes in the epithelial and stromal components. Am J Pathol. 2000;156:1093–8.
NCCN Clinical Practice Guidelines in Oncology: Breast Cancer Version 3.2019. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed Sept 2019
Wei J, Tan Y, Cai Y, et al. Predictive factors for the local recurrence and distant metastasis of phyllodes tumors of the breast: a retrospective analysis of 192 cases at a single center. Chin J Cancer. 2014;33:492–500.
Telli ML, Horst KC, Guardino AE, et al. Phyllodes tumors of the breast: natural history, diagnosis, and treatment. J Natl Compr Canc Netw. 2007;5:324–30.
Grabowski J, Salzstein SL, Sadler GR, et al. Malignant phyllodes tumors: a review of 752 cases. Am Surgeon. 2007;73:967–9.
Yabanoğlu H, Colakoglu T, Aytac HO, et al. Comparison of predictive factors for the diagnosis and clinical course of phyllodes tumours of the breast. Acta Chir Belg. 2015;115:27–32.
Shafi AA, AlHarthi B, Riaz MM, et al. Gaint phyllodes tumour with axillary & interpectoral lymph node metastasis; a rare presentation. Int J Surg Case Rep. 2020;66:350–5.
Morales-Vasquez J, Gonzalez-Angulo AM, Broglio K, et al. Adjuvant chemotherapy with doxorubicin and dacarbazine has no effect in recurrence-free survival of malignant phyllodes of the breast. Breast J. 2007;13:551–6.
Roberts N, Runk DM. Aggressive malignant phyllodes tumor. Int J Surg Case Rep. 2015;8:161–5.
Mituś JW, Blecharz P, Walasek T, et al. Treatment of patients with distant metastases from phyllodes tumor of the breast. World J Surg. 2016;40:323–8.
Yamamoto S, Yamagishi S, Kohno T, et al. Effective treatment of a malignant breast phyllodes tumor with doxorubicin-ifosfamide therapy. Case Rep Oncol Med. 2019;2759:650.
Yoshidaya F, Hayashi N, Takahashi K, et al. Malignant phyllodes tumor metastasized to the right ventricle: a case report. Surg Case Rep. 2015;1:121–5.
Acknowledgements
Not applicable.
Funding
There is no funding for this work.
Author information
Authors and Affiliations
Contributions
HA, AT, YT and YT performed the surgery and perioperative management on the patient, and HA, AT and YT drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent for publication of this case report and any accompanying images was obtained using the opt-out system.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abe, H., Teramoto, A., Takei, Y. et al. Malignant phyllodes tumor of the breast with rapid progression: a case report. surg case rep 6, 308 (2020). https://doi.org/10.1186/s40792-020-00986-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-020-00986-8