Abstract
Background
Adult-onset type II citurullinemia is an autosomal recessive disorder characterized by recurrent encephalopathy with hyperammonemia resulting from high plasma citrulline and ammonium levels. This report describes a rare case of adult-onset type II citurullinemia that occurred in a patient who only had the heterozygote mutation, and had never presented with any symptoms before surgery.
Case presentation
A 56-year-old man underwent a total gastrectomy for stomach cancer. On postoperative Day 13, he suddenly developed presyncope, and blood tests showed hyperammonemia and high levels of serum citrulline. He was diagnosed with hepatic encephalopathy. DNA analysis revealed a heterozygote mutation in Solute Carrier Family 25. Although the patient received a conservative treatment, episodes of loss of consciousness and abnormality of behavior repeatedly occurred.
Conclusion
Abdominal surgery involving the reconstruction of digestive tract alters the mechanisms of absorption and/or metabolism such that the symptoms of adult-onset type II citurullinemia may arise. Liver transplantation should be performed if all conservative treatments are unsuccessful.
Similar content being viewed by others
Background
Citrin deficiency is an autosomal disorder, and symptoms present during the childhood of most patients. Adult-onset type II citrullinemia 2 (CTLN2) is caused by mutations in the Solute Carrier Family 25 (SLC25A13) gene, which encodes a mitochondrial aspartate glutamate carrier protein. The symptoms of CTLN2 are often provoked by triggers such as alcohol, sugar intake, and surgery [1]. We report a rare clinical case of CTLN2, in which the patient had never experienced epileptic seizures or disturbances of consciousness until he underwent on a total gastrectomy.
Case presentation
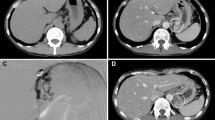
A laparoscopic-assisted total gastrectomy (final stage UICC 7th edition: T3N1M0, IIIB) was performed on a 56-year-old man to treat stomach cancer. On postoperative Day 13, the patient suddenly lost consciousness. Computed tomography showed no cerebral infarction and/or bleeding, and magnetic resonance imaging revealed no cerebral vascular accident, brain infarction, or encephalitis. Blood tests showed hyperammonemia (serum ammonia [NH3], 240 μg/dL; Table 1). He was diagnosed with hepatic encephalopathy and branched-chain amino acids (BCAAs) were administered. Two days after BCAAs administration, the serum NH3 level was reduced to 10% of hyperammonemic levels, and recovery of consciousness was observed. However, he was in an aggressive mental state for several days after recovery.
One month later, he received adjuvant chemotherapy (capecitabine + oxaliplatin) for gastric cancer. Two weeks after the start of the treatment, he experienced relapse of behavior disorder (serum NH3, 46 μg/dL). Electroencephalography demonstrated triphasic waves, accompanied with somewhat abnormal waves of metabolic abnormality. Two weeks later, he represented with loss of consciousness. Laboratory tests showed hyperammonemia (serum NH3, 858 μg/dL), and amino acid analysis showed hypercitrullinemia. Genomic DNA was extracted from his peripheral blood, and gene analysis revealed that he had a heterozygous 851del4 DNA nucleotide mutation in SLC25A13 (solute carrier family 25 member 13). Although the mutation was heterozygous, he was diagnosed with CTLN2 based on the mutation, recurrent symptoms, hyperammonemia, and hepatic encephalopathy.
Before surgery, the patient preferred eating nuts and fresh cream, but disliked sweat bean paste. However, his habitual dietary intake ceased after surgery, and he began to eat carbohydrate-rich foods such as noodles.
Although the patient received nutritional therapy with a carbohydrate-restricted, high protein/fat diet (total calories, 1800 kcal/day; protein: fat: carbohydrate ratio, 15:40:45), hepatic encephalopathy and/or aberrant behavior repeatedly developed twice in a week. Thereafter, the patient started dietary treatment with BCAAs, without receiving any other therapy such as arginine or sodium pyruvate administration. Finally, he was required to return to his normal eating, which consisted of nuts, cheese, fish, and small amount of carbohydrates. Although the frequency of hepatic encephalopathy and/or aberrant behavior episodes decreased, the symptoms still presented occasionally. Additionally, despite being nondiabetic and having a normal blood glucose level before the total gastrectomy, hyperglycemia (> 200 mg/dL) was sometimed observed in the patient after he developed CTLN2 symptoms. Liver transplantation, which is the only radical treatment for CTLN2, will be considered should his condition worsen.
Discussion
Citrin, a mitochondrial solute carrier protein encoded by SLC25A13, plays an important role in ureagenesis and gluconeogenesis. Citrin deficiency causes neonatal intrahepatic cholestasis (NICCD), failure to thrive and dyslipidemia caused by citrin deficiency (FTTDCD), and adult-onset type II citrullinemia (CTLN2) [1]. Citrin deficiency had been regarded as a hereditary disease in East Asia, but non-Asian cases have recently been reported. CTLN2 is an autosomal recessive disorder characterized by hyperammonemia and high plasma citrulline levels, accompanied by recurrent encephalopathy, delirium, abnormal behavior, seizures, and coma. The reduction of argininosuccinate synthetase activity in the urea cycle is regarded to cause of the symptoms [2,3,4,5,6].
Saheki and Kobayashi reported that approximately 1 in 70 people in Japanese population carry a mutation in one allele of the SLC25A13 [7]. Though the minimal estimated frequency of homozygotes (or compound heterozygotes) for SLC25A13 pathogenic variants is calculated to be 1 in 20,000, the incidence of CTLN2 is much lower, at 1 in 100,000 to 1 in 230,000 [1]. On the other hand, there are twice as many male CTLN2 patients as there are female, and such sex differences are not observed in NICCD patients [8]. There is a possibility that sex hormones affect enzymatic activity in the urea cycle, and can trigger CTLN2 symptoms even in patients with heterozygous mutations.
Diet also contributes to the occurrence of symptom, and patients tend to select an unbalanced protein- and lipid-rich diet. Komatsu et al. reported a correlation between the severity of hepatic steatosis and the down-regulation of free acid oxidation and proliferator-activated receptor alpha (PPARα), indicating that CTLN2 patients have low hepatic ATP levels; therefore, they prefer lipid-rich diets to generate more ATP through free acid oxidation [9].
Currently, conservative therapy for CTLN2 typically consists of a lipid- and protein-rich, low-carbohydrate diet, which may enhance mitochondrial β-oxidation activity and increase hepatic ATP levels [9]. However, sudden-onset symptoms, such as loss of consciousness and abnormal behavior, may be related to argininosuccinate synthase activity. Although administration of arginine and sodium pyruvate is also reported to improve hyperammonemia and delay the need for liver transplantation [10,11,12,13], the mechanism of action of this treatment remains unclear.
Alcohol, sugar intake, medication, and surgery are reported as precipitating factors of CTLN2 [1]. To the best of our knowledge, there are only two other cases reported in which CTLN2 occurred after surgical treatment (Table 2). One case was diagnosed at few days after total colectomy [14], while the other case was diagnosed 2 years after pancreaticoduodenectomy for duodenal malignant somatostatinoma [15]. Here, we report the third known case, diagnosed at 13 days after a total gastrectomy for stomach cancer as hepatic encephalopathy. The former two cases underwent liver transplantation after diagnosis of CTLN2. Typical conservative treatment consisting of nutritional therapy with a calorie/carbohydrate-restricted and protein-rich diet, was not effective in our case; the symptoms have occasionally recurred even after the patient returned to his normal eating habits. Reconstruction of the digestive tract is a common factor among these three patients, and indicates that the change in the mechanisms of nutrient absorption and/or metabolism may be a trigger of CTLN2. To supplement this notion, the plasma insulin level in diabetic rats is significantly reduced in Roux-en-Y reconstruction after total gastrectomy [16]. Consistent with this, hyperglycemia was occasionally observed in the present case after the symptoms appeared, even though the patient was non-diabetic and had a normal preoperative blood glucose level. Therefore, it follows that reconstruction of the digestive tract increases the susceptibility to the development of hyperglycemia, which is a risk factor of disease onset.
When several conservative treatments fail, liver transplantation, which is a practical treatment that fundamentally improves the patient’s quality of life, should be performed. Kimura et al. reported that the survival rate in CTLN2 patients treated with liver transplantation was 100%, but was 76.5% in patients who received conservative treatment [11]. Conservative treatment did not yield sufficient results in the present case, which indicated that the abdominal surgery requiring digestive tract reconstruction may exhibit resistance to conservative treatment. Liver transplantation, which is the only established radical treatment for CTLN2, will be necessary if his condition worsens; however, uncontrollable malignant tumors are a contraindication for liver transplantation. In particular, tumor recurrence or metastasis will be problematic due to his final pathological stage. Recently, a meta-analysis revealed that immunosuppressive therapies did not increase the incidence of tumor recurrence [17]. While uncontrollable malignant tumors contraindicate liver transplantation, it may be difficult to continue adjuvant chemotherapy without controlling the patient’s CTLN2 symptoms. Thus, although it is important to carefully assess the criteria and the timing, it is essential to consider treatment with liver transplantation without waiting for follow-up period of 5-year recurrence-free survival if all conservative therapies fail. Further investigation of novel conservative therapies is necessary to provide a safer, and more affordable, as an alternative to liver transplantation in the near future.
Conclusion
Abdominal surgery requiring the reconstruction of the digestive tract alters metabolic mechanisms, such that CTLN2 symptoms may arise even in heterozygotic patients. When conservative CTLN2 treatment fails, liver transplantation, which is an established radical procedure, should be performed.
Abbreviations
- ATP:
-
Adenosine triphosphate
- CTLN II:
-
Adult-onset type II citrullinemia
- SLC25A13:
-
Solute carrier family 25 member 13
References
Kobayashi K, Saheki T. Citrin Deficiency. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2005 Sep 16 [Updated 2008 Jul 01]. https://www.ncbi.nlm.nih.gov/books/NBK1181/.
Palmieri L, Pardo B, Lasorsa FM. Citrin and aralar1 are Ca2+−stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001;20(18):5060–9.
Begum L, Jalil MA, Kobayashi K. Expression of three mitochondrial solute carriers, citrin, aralar1 and ornithine transporter, in relation to urea cycle in mice. Biochim Biophys Acta - Gene Struct Expr. 2002;1574(3):283–92.
Saheki T, Kobayashi K, Iijima M. Pathogenesis and pathophysiology of citrin (a mitochondrial aspartate glutamate carrier) deficiency. Metab Brain Dis. 2002;17:335–46.
Palmieri F. The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Asp Med. 2013;34(2–3):465–84.
Nagata N, Matsuda I, Oyanagi K. Estimated frequency of urea cycle enzymopathies in Japan. Am J Med Genet. 1991;39(2):228–9.
Saheki T, Kobayashi K. Mitochondrial aspartate glutamate carrier (citrin) deficiency as the cause of adult-onset type II citrullinemia (CTLN2) and idiopathic neonatal hepatitis (NICCD). J Hum Genet. 2002;47:333–41.
Yamaguchi N, Kobayashi K, Yasuda T, Nishi I, Iijima M, Nakagawa M, Saheki T. Screening of SLC25A13 mutations in early and late onset patients with citrin deficiency and in the Japanese population: identification of two novel mutations and establishment of multiple DNA diagnosis methods for nine mutations. Hum Mutat. 2002;19(2):122–30.
Komatsu M, Kimura T, Yazaki M. Steatogenesis in adult-onset type II citrullinemia is associated with down-regulation of PPARα. Biochim Biophys Acta - Mol Basis Dis. 2015;1852(3):473–81.
Yazaki M, Ikeda S, Kobayashi K, Saheki T. Therapeutic approaches for patients with adult-onset type II citrullinemia (CTLN2): effectiveness of treatment with low-carbohydrate diet and sodium pyruvate. Rinsho Shinkeigaku. 2010;50:844–7.
Kimura N, Kubo N, Narumi S. Liver transplantation versus conservative treatment for adult-onset type II citrullinemia: our experience and a review of the literature. Transpl Proc. 2013;45(9):3432–7.
Honda S, Yamamoto K, Sekizuka M. Successful treatment of severe hyperammonemia using sodium phenylacetate powder prepared in hospital pharmacy. Biol Pharm Bull. 2002;25(9):1244–6.
Fukushima K, Yazaki M, Nakamura M. Conventional diet therapy for hyperammonemia is risky in the treatment of hepatic encephalopathy associated with citrin deficiency. Intern Med. 2010;49(3):243–7.
Eriguchi Y, Yamasue H, Doi N. A case of adult-onset type II citrullinemia with comorbid epilepsy even after liver transplantation. Epilepsia. 2010;51(12):2484–7.
Tazawa KI, Yazaki M, Fukushima K, Ogata S, Makuuchi M, Morita K, Hiraishi H, Iwasaki Y, Kubota K, Ikeda S. Patient with adult-onset type II citrullinemia beginning 2 years after operation for duodenal malignant somatostatinoma: indication for liver transplantation. Hepatol Res. 2013;43(5):563–8.
Zhou D, Jiang X, Jian W, Zheng L, Lu L, Zheng C. Comparing the effectiveness of total gastrectomy and gastric bypass on glucose metabolism in diabetic rats. Obes Surg. 2016;26(1):119–25.
Shelton E, Laharie D, Scott FI, Mamtani R, Lewis JD, Colombel JF, Ananthakrishnan AN. Cancer recurrence following immune-suppressive therapies in patients with immune-mediated diseases: a systematic review and meta-analysis. Gastroenterol. 2016;151(1):97–109.e4.
Funding
None.
Author information
Authors and Affiliations
Contributions
RK designed the study and wrote the initial draft of the manuscript. RK and KM performed the surgery and organized the writing of the manuscript. AW assisted in the preparation of the manuscript. KM, TH, TK, and SA contributed in the critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent was obtained from the patient for the publication of this case report and any accompanying figures.
Competing interest
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Komine, R., Minamimura, K., Watanabe, A. et al. Sudden development of adult-onset type II citrullinemia after total gastrectomy: a case report. surg case rep 4, 11 (2018). https://doi.org/10.1186/s40792-018-0420-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-018-0420-9