Abstract
Background
Mosses dominate much of the vegetation in the Antarctic, but the effect of climatic change on moss growth and sexual reproduction has scarcely been studied. In Antarctica, mosses infrequently produce sporophytes; whether this is due to physiological limitation or an adaptive response is unknown. We studied the effect of experimental warming (with Open Top Chambers, OTCs) on sporophyte production on Fildes Peninsula, King George Island for four moss species (Bartramia patens, Hennediella antarctica, Polytrichastrum alpinum, and Sanionia georgicouncinata). To determine whether reducing cold stress increases sexual reproduction as would be predicted if sex is being constrained due to physiological limitations, we counted sporophytes for these four moss species in OTC and control plots during two years. Also, we measured sporophyte size for a smaller sample of sporophytes of two species, B. patens and H. antarctica, in the OTC and control plots.
Results
After 2 years of the experimental treatment, maximum daily air temperature, but not daily mean air temperature, was significantly higher inside OTCs than outside. We found a significant species by treatment effect for sporophyte production, with more sporophytes produced in OTCs compared with controls for B. patens and P. alpinum. Also, sporophytes of B. patens and H. antarctica were significantly larger in the OTCs compared with the control plots.
Conclusions
Our results suggest that the lack of sexual reproduction in these Antarctic mosses is not adaptive but is constrained by current environmental conditions and that ameliorating conditions, such as increased temperature may affect sexual reproduction in many Antarctic mosses, altering moss population genetics and dispersal patterns.
Similar content being viewed by others
Background
The Antarctic Peninsula and the Scotia Arc region of the Southern Ocean (including the South Orkney Islands, Elephant Island, and the South Shetland Islands) are among the fastest warming regions on Earth [9, 64, 65]. Records show an increase of 0.2 °C per decade since the 1950s in the Scotia Arc region (e.g., South Orkney Islands) and an even greater increase of 0.56 °C on the western side of the Antarctic Peninsula (Faraday/Vernadsky research stations; [63]). In some regions, such as on the western Antarctic Peninsula, temperature increases have been highest in autumn and winter [51], before the main growing season starts, while in other regions, such as on the eastern Antarctic Peninsula, summer warming has been greatest [60, 64]. Whereas the warming trend along the Antarctic Peninsula is supported by a 50-year record, few long-term data exists for precipitation because in situ measurement of precipitation on the Antarctic continent is difficult; much of the knowledge of precipitation variability has been derived from ice cores [63]. Using this kind of information, Monaghan et al. [37] showed no statistical change in Antarctic ice accumulation across the continent since the middle of the last century. However, at the Faraday/Vernadsky stations, a positive trend in the number of annual precipitation days has been measured, with an increase of 12.4 days decade−1 since the 1950s, with the majority of that increase occurring during the summer-autumn season (Turner et al. [62]). Consequently, there has been changes in soil water availability based on this increase in precipitation as well as from melting of glaciers, especially during the Antarctic summer. Biodiversity in Antarctica is strongly driven by patterns of water availability [19], and the increase in water availability with climate changes will thus likely alter patterns of diversity, and expose new potential habitats to be colonized by terrestrial biota, particularly pioneers such as lichens and bryophytes [17, 18, 61].
The Antarctic vegetation is dominated by a cryptogamic flora, with numerous species of lichens and bryophytes. The bryophytes include ca. 112 species of mosses and 27 species of liverworts along the maritime Antarctic [40, 54]. The effect of climate warming on bryophytes in stressful habitats has been studied extensively in other parts of the world, such as in the alpine and in the Arctic. Elmendorf et al. [23] analyzed 61 experimental warming studies on tundra vegetation in the alpine and Arctic and found that mosses were the most negatively impacted element of the vegetation with acrocarpous mosses (similar to those in Antarctica) much more affected than pleurocarpous mosses (which are more common in the Arctic). However, these studies of passive warming have principally measured plant cover, biomass or growth, with no data available on the impact of warming on moss reproduction.
To date little is known about the responses of Antarctic mosses to climate change [44]. For Antarctic mosses, it has been recently shown that growth rates have declined since 1980 in East Antarctica, at sites near Windmill Islands and Vestfold Hills [14], and that this response is due to lower water availability caused by increasing temperature and wind speed during the last 50 years. Experiments in Open Top Chambers (OTCs) carried out in three different locations on the Falkland, Signy, and Anchorage Island on cryptogamic communities showed no significant effect of warming on mosses [5]. In contrast, in situ experiments by Day et al. [20, 21] in vascular plant-dominated communities have determined a decrease in moss cover after 4 years of long term growth under passive warming on Anvers Island, along the Antarctic Peninsula. In these experiments, it is unclear whether warming directly decreases moss cover or whether increases in vascular plant cover caused by warming leads indirectly to decreases in moss cover. Hill et al. [29] suggest that mosses are likely to be outcompeted by the grass Deschampisia antarctica as soils warm due to the increase in the decomposition rate of organic matter resulting in larger pools of proteinaceous nitrogen, and the more efficient acquisition by vascular plants of nitrogen from protein decomposition. However, earlier warming experiments using passive warming suggest that on bare substrate without plants, moss cover increased by 40 % in 2 years [32]. Also, in the maritime Antarctic, the vertical accumulation rates of Chorisodontium aciphyllum peat moss have increased during the last two century, suggesting regionalwarming is increasing moss growth rates [45]. While these studies and others have shown that warming affects growth rates for Arctic and Antarctic bryophyte systems, there is virtually no data available on the effects of warming on bryophyte reproduction or phenology.
Rates of sexual reproduction in bryophytes generally decrease with increasing latitude ([16, 36]; but see [53]), suggesting temperature is a primary driver of sexual reproduction in bryophytes. Sporophytes (the diploid product of sexual reproduction in bryophytes) are produced on 80–90 % of species of Guatemalan and New Zealand mosses; 76 % of the British Island moss flora have been recorded with sporophytes; and fewer than 25 % of Antarctic moss species have been found with sporophytes (see [16]). In Antarctica, temperature generally correlates with rates of bryophyte sexual reproduction [50]; between 25 and 33 % of bryophytes have sporophytes in the maritime Antarctica while in continental Antarctica sexual reproduction is extremely rare with only 10 % of bryophytes producing sporophytes [16, 49, 54]. At finer scale resolution there appears to be a microclimatic effect. Studies in the south maritime Antarctic (along 68–72° LS) have shown that a high percent of Antarctic moss species (43 % in Marguerite Bay area and 47 % in Alexander Island) produce sporophytes in so called “favorable small scale oases,” and those that do produce sporophytes regularly invest heavily, both in sporophyte biomass and number [16, 53, 67].
Reduced sexual reproduction in mosses may be due to adaptation or physiological limitation. One possibility is that the mosses of Antarctica are under selective pressure to reproduce asexually rather than via sexual reproduction, perhaps because only a few phenotypes are adapted to such environments. Under such a scenario, sexual reproduction would not be adaptive under extreme stress and individuals that have evolved to favor asexual reproduction would be favored [33]. Alternatively, the abiotic conditions of Antarctica may limit sexual reproduction via short growing seasons, sporophyte mortality due to desiccation [67], sporophyte abortion after extreme conditions in winters or summers [26, 67], and notably diurnal freeze-thaw cycles which may prevent gametangial initiation or maturation, fertilization, or sporophyte development [35]. Furthermore, in species with separate sexes, one sex may be less stress tolerant than the other sex (e.g., [57, 66]), altering the population sex ratio and reducing the probability of sexual reproduction.
Here, we tested the effects of passive warming experiments on sexual reproduction in Antarctic moss communities on Fildes Peninsula in the maritime Antarctica on King George Island (KGI). We selected four moss species growing at two study sites, considering both sexual systems because we were interested in how warming would influence sexual reproduction in dioecious and monoecious species and the differential responses of perennial versus short-lived species. We used Open Top Chambers (OTCs) of a hexagonal chamber model for in situ passive warming, as these are the most suitable for experimental warming studies in the Antarctica [6]. We report the change in sporophyte production in four moss species in OTCs and control plots after 2 years. If physiological limitations rather than adaptation are limiting sexual reproduction, we predict that experimental warming will increase sexual reproduction in Antarctic mosses and that this response will be species-specific.
Methods
Study site
The study was carried out on Fildes Peninsula, King George Island (62° 00’S, 58° 15’W) in the South Shetland Island Archipelago. Bryophytes cover large areas (>100 m2) mainly within 200 m of the coast and in depressions, where moss communities are well-developed, extending several hundred meters on Collins Bay, Nebles Point, and Valle Grande. In total, 61 moss species have been recorded on King George Island, of which 40 are present on Fildes Peninsula, one of the largest ice-free areas on the South Shetland Island Archipelago [39]. The experiments were carried out at two sites, Juan Carlos Point (62°12’ S 58°59’W, 37 m a. s. l.) and La Cruz Plateau (62°12’S, 58°57’ W, 41 m a. s. l.) (Fig. 1). Juan Carlos Point, which is characterized by northern exposure towards Drake Passage, has a moss-grass community dominated by the grass Deschampsia antarctica Desv and two to three moss species (frequently Sanionia spp.), and this community is found on several islands along the South Shetland Archipelago [13]. La Cruz Plateau is located in the interior of Fildes Bay, which is oriented towards Bransfield Strait, and characterized by polygonal soils with permafrost about 90 cm deep. La Cruz Plateau has a moss-lichen community dominated by the lichens Usnea aurantiacoatra (Jacq.) Bory and Himantormia lugubris (Hue) I.M. Lamb.
Study species
Fildes Peninsula is the second largest ice-free area along the western Antarctic Peninsula (Olech [41]). Non-vascular cryptogamic vegetation dominates, and the only vascular plant to grow on Fildes Peninsula is the grass Deschampsia antarctica Desv. The climate of the Fildes Peninsula is mild by Antarctic standards, with a maritime climate in the summer and polar conditions in the winter [11]. From 1970 to 2004, mean daily air temperature during the growing season (December-February) was between 0.6 and 1.5 °C, and the lowest mean daily air temperature in winter (July-August) was −6.5 °C [11]. From 1970 = 2004, Fildes Peninsula was overcast more than 70 % of the time in any month, and summer rain was common with mean monthly rain between 40 to 70 mm (January-February; [11]).
There are approximately 109 lichens and 40 bryophytes on the Fildes Peninsula [1, 40]. We selected four moss species: 1) Polytrichastrum alpinum (Hedw.) G.L. Sm., which is dioecious (the most common sexual system in mosses; [68]) and 2) Sanionia georgicouncinata (Hedw.) Loeske, 3) Bartramia patens Brid., and 4) Hennediella antarctica (Ångström) Ochyra & Matteri, which are all monoecious. The long-lived species P. alpinum and S. georgicouncinata rarely reproduce sexually in Antarctica [40]. The short-lived species, B. patens and H. antarctica, produce sporophytes frequently on subantarctic islands and on the South Shetland Island Archipelago, where H. antarctica can colonizes areas of several square meters [40]. In contrast, on Fildes Peninsula the two monoecious species grow in small and disperse patches of about two cm in diameter, frequently as pioneers on the moraine of glaciers in rock crevices or growing in moss-lichen communities. Dried reference samples of identified moss species were deposited at the Herbarium of Concepción University (CONC).
Passive warming experiments
In 2008, we installed a warming experiment on Fildes Peninsula, King George Island. Ten Open Top Chambers (OTCs) and ten control plots were installed at each of the two sites (La Cruz Plateau and Juan Carlos Point). The chambers were designed to produce an increase in air temperature by preventing the loss of heat by convection and have been used in other ecosystems, such as the Arctic tundra for many years [28]. The OTCs used are similar to those previously installed elsewhere in Antarctica [5, 6]; they are hexagonally sided, tapering to an open top and assembled of 3 mm thick, transparent acrylic panels of 40 cm height, with a basal area of 106.4 cm2. The acrylic walls have small perforations to allow better air exchange and hence avoid excessive warming. There are ten control plots at each site, each one assigned to a nearby OTC, having a similar floristic composition to each OTC and approximately 80–90 % plant cover (with moss cover approximately 50 % and the remainder lichen). To characterize microclimatic differences produced by the OTCs, air temperature and relative humidity measurements were taken both inside the OTCs and in the control plots using HOBO Pro v2 loggers (Onset, Bourne, Mass.) programmed to record temperature every hour throughout the year. Sensors were placed at 20 cm above the vegetation inside two OTCs and in two control plots. For temperature effects, we analysis all monthly air temperature values (from February 2008 to March 2010), but for relative humidity we used only values for the spring–summer season (November to March), as this included the majority of time when temperatures were above freezing and mosses would be physiologically active. We recognize that OTCs can alter temperature and snow conditions in other seasons [6, 7], potentially causing physiological effects in the mosses.
Sporophyte measures
Sporophyte production was quantified for all four moss species, in two consecutive summers (2008–2009 and 2009–2010); the number of sporophytes was recorded in situ for each moss species in whole plots, for all ten OTC and control plots at both sites (La Cruz Plateau and Juan Carlos Point). However, S. georgicouncinata never produced sporophytes during the experimental period and thus was not included in the statistical analysis for sporophyte production. In 2010, for two species, H. antarctica and B. patens, we also conducted more intense sporophyte sampling on smaller areas (about 2 cm2) within plots. From these sub-samples, for H. antarctica and B. patens, lengths of sporophytes, sporophyte capsules, and setae were recorded. Only ten H. antarctica and five B. patens individuals were harvested (per treatment) for sporophyte size measurements, as so few sporophytes were produced. Additionally, to minimize plot damage, we could not quantify the number of moss stems per species as this would impede ongoing long-term experiments within the chambers.
Statistical analyses
To determine the effects of treatment (OTC and control), site (La Cruz Plateau and Juan Carlos Point), and interactions between these two factors on temperature and humidity measures, we used a series of ANOVA, using Infostat [22]. To determine the effect of species (Bartramia patens, Hennediella antarctica, and Polytrichastrum alpinum), treatment (OTC and control), site (La Cruz Plateau and Juan Carlos Point), and interactions among these effects on sporophyte production over 2 years, we used a generalized linear model with a Poisson distribution, using JMP [48], and post hoc tests, using Infostat [22]. We used Akaike Information Criterion (AIC) and overdispersion analysis to evaluate potential models and determine which interactions to include [27, 42]. We used an ANOVA to determine the effects of treatment (OTC and control), species (H. antarctica and B. patens), and interactions between these factors on whole sporophyte length, capsule length, and seta length using Infostat [22].
Results
Warming with Open Top Chambers (OTCs)
The OTC treatments increased mean maximum daily air temperature during the study period (2008–2010), from 7.3 °C in control plots to 10.5 °C (Table 1a;F1,97 = 5.78, p < 0.018). However, warming treatment had no significant effect on mean daily temperature or mean minimum daily air temperature (Table 1). Site significantly affected the mean minimum daily air temperature (F1,100 = 5.92, p < 0.0168), being lower at La Cruz Plateau (−9.42 °C) compared to Juan Carlos Point (−6.4 °C; Table 1b). The interaction between treatment and site was not significant for any of the abiotic measures. The highest warming effect we measured was during the summer season, with an increase of 0.61 °C inside the OTCs compared to the control plots [12]. The temperature changes we recorded between treatments were similar to values reported in warming experiments for the Antarctic area, where Bokhorst et al. [5] measured an increase of 0.7 °C in annual mean temperature inside OTCs in comparison with the control plots.
The use of OTCs not only affected the air temperature, but also produced additional changes in microclimate. In general, mean daily relative humidity was significantly lower in the OTCs (80.7 %) compared to the controls (91.7 %; Table 1a). This difference occurred across treatments at both research sites (data not shown), despite the fact that the La Cruz Plateau and Juan Carlos Point sites differed overall in mean daily relative humidity (83.9 % and 88.5 %, respectively, Table 1b).
Moss responses to Open Top Chambers
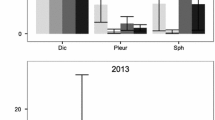
Our results provide the first evidence that experimental warming treatments generally have a positive effect on sexual reproduction in several Antarctic mosses. We found that the number of sporophytes in plots was significantly affected by treatment, species, and the two-way interaction between treatment and species (Table 2a). The three moss species (B. patens, H. antarctica, and P. alpinum, which produced sporophytes during our experiment) differed in their level of sporophyte production (Table 3), and responded differentially to the OTC treatments, with two species (P. alpinum and B. patens) showing greater sporophyte production in the OTCs compared with the controls, and one species (H. antarctica) showing no response to the treatment (Table 3). Sporophyte production with our experimental warming treatment was 90.6 % greater for B. patens than in controls and for P. alpinum was present in warmed plots while absent in controls in both sites. Site as well as the interaction between site and species had significant effects on sporophyte production (Tables 2a and 3). There was greater sporophyte production at the La Cruz Plateau site (75 % of plots had sporophytes) compared with the Juan Carlos Point site (only 15 % of plots had sporophytes), and this difference was significantly species-specific (Tables 2a and 3).. The fourth moss species for which we measured sporophyte production, S. georgicouncinata, did not produce sporophytes in any OTC or control plots (Table 3). Additionally, sporophyte size was significantly increased in both B. patens and H. antarctica growing inside the OTCs compared with those in the control plots (Table 2b, Fig. 2)
Discussion
Sexual reproduction and the production of sporophytes in mosses may be reduced by sperm-limitation [43, 46], resource limitation [24, 56, 58, 59], and abiotic stress ([8]; Eppley et al. [25]). Our experimental warming treatments increased sporophyte production in two moss species, P. alpinum and B. patens, compared with controls (Tables 2a and 3), and this warming potentially altered many steps in the process of sporophyte formation, from sperm and egg production, to gamete dispersal, to fertilization success, to sporophyte maturation. Warming has the potential to decrease abiotic stress, freeing up resources used for stress defense (e.g., [38]); alter resource availability by shifting the carbon balance and/or nutrient cycling (e.g., [47]); and ultimately reduce sperm-limitation by increasing the number of males, antheridial initiations, and successful dispersal and fertilization events (all of which are often low in Antarctic mosses; [36]).
We hypothesize that an important mechanism in the greater number of sporophytes in our warming treatments compared with controls for these two species is that the increase in temperature caused by OTCs alters the carbon balance for the plants, potentially increasing the rate of photosynthesis to the point where plants are producing sufficient carbohydrates both for respiration (which may also alter with warming see [2]) and additional sporophyte production. Increased primary productivity has been observed under elevated temperatures in three Antarctic moss species ([52]; but see [31]), indicating that temperature is limiting photosynthesis in some but not all Antarctic moss species. Thus, there is the potential for additional carbohydrate gain with increased temperature in some species. Sexual reproduction, including sporophyte formation is thought to be quite costly in bryophytes, taking at least 15 % of gametophytic biomass [3, 34], and thus the ability of these species to have additional resources for sporophyte formation could be the tipping point for species to invest in sporophyte production.
In fact, we observed that sporophyte size was increased significantly in both B. patens and H. antarctica growing inside the OTCs compared with those outside (Table 2a, Fig. 2). The results suggest the potential that the plants in these species had additional carbohydrates available to invest in larger sporophytes. Larger sporophytes are correlated with more spores and higher fitness [10], and sporophytes with longer setae are able to vibrate and thus release pollen for longer dispersal at lower wind speeds than those with shorter setae [30]. Consequently, the investment in larger sporophytes that we measured in the two Antarctic mosses can potentially generate benefits in the colonization of new ice-free areas under a regional change scenario.
We observed that sporophyte production is sparse and patchy in the Antarctic fellfield. Mosses at the La Cruz Plateau site produced more sporophytes (75 % of plots had sporophytes) compared with those at the Juan Carlos Point site (only 15 % of plots had sporophytes), suggesting that microclimatic characteristics affect the reproductive output in these mosses. At the species level, P. alpinum produced sporophytes only at la Cruz Plateau under warming and B. patens increased the production of sporophytes consistently with warming at both sites (from 0 to 0.17 ± 0.1 in Juan Carlos Point and 0.7 ± 0.4 to 7.3 ± 3.4 at La Cruz Plateau). On the other hand, H. antarctica produced the majority of sporophytes at Juan Carlos Point and decreased production at La Cruz Plateau (9.3 ± 5 in controls compared to 5.5 ± 3.4 in OTCs), which could be due to the decrease in mean daily relative humidity as a consequence of warming induced by the OTCs (Table 1). The most important microclimatic difference between the two sites is likely the lower temperature at La Cruz Plateau (Table 1) and that there is permafrost at around 90 cm at this site, which should influence water availability at the site, improving the performance of H. antarctica in the control plots compared to the OTCs. Also, the increase in sporophytes with OTCs did not occur consistently across sites for the three species with high sporophyte production (Tables 2a and 3). For example, in B. patens the increase in sporophyte production in OTCs compared to controls was greater at La Cruz Plateau than Juan Carlos Point, where control plots had no sporophytes at all. In H. antarctica, sporophytes did not increase in OTCs at either site, and actually decreased slightly at La Cruz Plateau (Table 3). Smith & Convey [53] found that in southern maritime Antarctica (68–72°S) almost half of the bryophyte species could produce sporophytes at sites where a favorable microclimate generates available niches, suggesting that micro-site differences matter in Antarctica for bryophyte reproduction and that climate stress may limit sexual reproduction in Antarctic bryophytes. Our experiment supports this result as a slight microclimate improvement (increase in temperature) induced a greater sexual response in mosses in the colder La Cruz plateau compared with the warmer Juan Carlos Point site.
While our OTCs are designed to increase temperature and were successful in this regard, it is also important to acknowledge that the OTCs change not only temperature, but other variables including relative humidity, which is likely to be equally important to sporophyte production [10]. Humidity decreased in the OTCs, and while generally moss sexual reproduction responds poorly to decreased relative humidity and water availability, there are rare instances where this is not the case [55]. Also, the increases in sporophytes we recorded may have been caused by an increase in many stages during sexual reproduction from gametangia production, gamete production, and fertilization success to sporophyte formation. While we have focused on sporophyte production in this first analysis, future work needs to assess all stages in the moss reproductive cycle to determine whether earlier steps might be limiting sexual reproduction in these Antarctic mosses.
Conclusion
This is the first study of sexual reproduction in mosses under experimental warming conditions in Antarctica. Our data show that field experimental warming enhances sexual reproduction in some, but not all, moss species. These results suggest that warming may improve investment in sexual reproduction in mosses, and support previous predictions that the effects of climate change on Antarctic terrestrial biota have the potential to be positive. Block et al. [4] predicted that in the short term the majority of the terrestrial Antarctic fellfield biota would be able to absorb the effects of a changing climate because of the high levels of physiological tolerance and life-cycle flexibility common to these species, and Convey [15] suggests that warming will enhance Antarctic terrestrial biota, although human disturbance and invasives are likely to have an increasing negative impact. Our results suggests that different moss species will respond differently to climate change in Antarctica, and understanding these species-specific responses in bryophytes will be critical to understanding plant responses to climate change in Antarctica. Future work in Antarctic bryophytes should focus on understanding how temperature affects gametangia and sporophyte production across light and humidity levels in each species.
Abbreviations
- OTC:
-
Open top chamber
References
Andreyev MP. The lichens in the vicinity of Bellingshausen station, King George Island. Polar Geograph Geol. 1989;13:42–5.
Atkins OK, Bruhn D, Hurry VM, Tjoelker MG. The hot and the cold: unravelling the variable response of plant respiration to temperature. Funct Plant Biol. 2005;32:87–105.
Bisang I, Ehrlén J. Reproductive effort and cost of sexual reproduction in female Dicranum polysetum. Bryol. 2002;105:384–97.
Block W, Smith RIL, Kennedy AD. Strategies of survival and resource exploitation in the Antarctic fellfield ecosystem. Biol Rev Camb Phil Soc. 2009;84:449–84.
Bokhorst S, Huiskes A, Convey P, Aerts R. The effect of environmental change on vascular plant and cryptogamic communities from the Falkland Islands and the Maritime Antarctic. BMC Ecol. 2007;7:15.
Bokhorst S, Huiskes A, Convey P, Sinclair BJ, Lebouvier M, Van de Vijver B, Wall DH. Microclimate impacts of passive warming methods in Antarctica: implications for climate change studies. Polar Biol. 2011;34:1421–35.
Bokhorst S, Huiskes A, Aerts R, Convey P, Cooper EJ, Dalen L, Erschbamer B, Gudmundsson J, Hofgaard A, Hollister RD, Johnstone J, Jónsdóttir IS, Lebouvier M, van de Vijver B, Wahren CH, Dorrepaal E. Variable temperature effects of Open Top Chambers at polar and alpine sites explained by irradiance and snow depth. Global Change Biol. 2013;19:64–74.
Bowker MA, Stark LR, McLetchie DN, Mishler BD. Sex expression, skewed sex ratios, and microhabitat distribution in the dioecious desert moss Syntrichia caninervis (Pottiaceae). Am J Bot. 2000;87:517–26.
Bracegirdle TJ, Connolley WM, Turner J. Antarctic climate change over the twenty first century. J Geophys Res. 2008;113:D03103. doi:10.1029/2007JD008933.
Budke JM, Goffinet B, Jones CS. Dehydration protection provided by a maternal cuticle improves offspring fitness in the moss Funaria hygrometrica. Ann Botany. 2013;111:781–9.
Carroscao J, González M. Climatología de la Peninsula Antártica y de la base Presidente Eduardo Frei Montalva. Dirección General de Aeronáutica Civil. Dirección Meteorológica de chile; 2007. http://164.77.222.61/climatologia/publicaciones/Climatologia_Frei.pdf.
Casanova-Katny A, Pizarro M, Caballero MM, Cordero R, Zúñiga GE. Non-structural carbohydrate content in cryptogamic Antarctic species after two years of passive warming on the Fildes Peninsula. Czech Polar Rep. 2015;5:88–98.
Casanova-Katny MA, Cavieres LA. Antarctic moss carpets facilitate growth of Deschampsia antarctica but not its survival. Polar Biol. 2012;35:1869–78.
Clarke LJ, Robinson SA, Hua Q, Ayre DJ, Fink D. Radiocarbon bomb spike reveals biological effects of Antarctic climate change. Global Change Biol. 2012;18:301–10.
Convey P. Antarctic terrestrial biodiversity in a changing world. Polar Biol. 2011;34:1629–41.
Convey P, Smith RIL. Investment in sexual reproduction by Antarctic mosses. Oikos. 1993;68:293–302.
Convey P, Smith RIL. Responses of terrestrial Antarctic ecosystems to climate change. Plant Ecol. 2006;182:1–10.
Convey P, Bindschadler R, Di Prisco G, Fahrbach E, Gutt J, Hodgson DA, Mayewski PA, Summerhayes CP, Turner CP. Antarctic climate change and the environment. Antarct Sci. 2009;21:541–63.
Convey P, Chown SL, Clarke A, Barnes DKA, Bokhorst S, Cummings V, Ducklow HW, Frati F, Green TGA, Gordon S, Griffiths HJ, Howard-Williams C, Huiskes AHL, Laybourn-Parry J, Lyons WB, McMinn A, Morley SA, Peck LS, Quesada A, Robinson SA, Schiaparelli S, Wall DH. The spatial structure of Antarctic biodiversity. Ecol Monogr. 2014;84:203–44.
Day TA, Ruhland CT, Xiong FS. Warming increases aboveground plant biomass and C stocks in vascular-plant-dominated Antarctic tundra. Global Change Biol. 2008;14:1827–43.
Day TA, Ruhland CT, Strauss SL, Park JH, Krieg ML, Krna MA, Bryant DM. Response of plants and the dominant microarthropod, Cryptopygus antarcticus, to warming and contrasting precipitation regimes in Antarctic tundra. Global Change Biol. 2009;15:1640–51.
Di Rienzo J, Casanoves F, Balzarini M, Gonzalez L, Tablada M, Robledo C. InfoStat versión 2014. Grupo InfoStat, FCA. Argentina: Universidad Nacional de Córdoba; 2014.
Elmendorf SC, Henry GHR, Hollister RD, Bjork RG, Bjorkman AD, Callaghan TV, Collier LS, Cooper EJ, Cornelissen JHC, Day TA, Fota AM, Gould WA, Gretarsdottir J, Harte J, Hermanutz L, Hik DS, Hofgaard A, Jarrad F, Jonsdottir IS, Keuper F, Klanderud K, Klein JA, Koh S, Kudo G, Lang SI, Loewen V, May JL, Mercado J, Michelsen A, Molau U, Myers-Smith IH, Oberbauer SF, Pieper S, Post E, Rixen C, Robinson CH, Schmidt NM, Shaver GR, Stenstrom A, Tolvanen A, Totland O, Troxler T, Wahren CH, Webber PJ, Welker JM, Wookey PA. Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecol Lett. 2012;15:164–75.
Ehrlén J, Bisang I, Hedenäs L. Costs of sporophyte production in the moss, Dicranum polysetum. Plant Ecol. 2000;149:207–17.
Eppley SM, Rosenstiel TN, Graves CB, Garcia E. Limits to sexual reproduction in geothermal bryophytes. Int J Plant Sci. 2011;172:870–978.
Greene SW. Bryophyte distribution. In: Bushnell VC, editor. Terrestrial life of Antarctica. Am Geograp Soc. New York; 1967. p. 11–13.
Guisan A, Edwards TC, Hastie T. Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecol Model. 2002;157:89–100.
Henry GHR, Molau U. Tundra plants and climate change: the International Tundra Experiment (ITEX). Global Change Biol. 1997;3:1–3.
Hill PW, Farrar J, Roberts P, Farrell M, Grant H, Newsham KK, Hopkins DW, Bardgett RD, Jones DL. Vascular plant success in a warming Antarctic may be due to efficient nitrogen acquisition. Nat Clim Chang. 2011;1:50–3.
Johansson V, Lonnell N, Sundberg S, Hylander K. Release thresholds for moss spores: the importance of turbulence and sporophyte length. J Ecol. 2014;102:721–9.
Kappen L, Smith RIL, Meyer M. Carbon dioxide exchange of 2 ecodemes of Schistidium antarctici in continental Antarctica. Polar Biol. 1989;9:415–22.
Kennedy AD. Antarctic terrestrial ecosystem repsonse to global environmental change. Ann Rev Ecol Sys. 1995;26:683–704.
Kis-Papo T, Kirzhner V, Wasser SP, Nevo E. Evolution of genomic diversity and sex at extreme environments: Fungal life under hypersaline Dead Sea Stress. PNAS. 2003;100:14970–5.
Laaka-Lindberg S. Biomass allocation to sexual and asexual reproduction in a leafy hepatic Lophozia silvicola Buch. J Bryol. 2001;23:3–8.
Longton RE. Reproductive biology and evolutionary potential in bryophytes. J Hatt Bot Lab. 1976;41:205–23.
Longton RE. Biology of Polar Bryophytes and Lichens. Cambridge: Cambridge University Press; 1988.
Monaghan AJ, Bromwich DH, Fogt RL, Wang SH, Mayewski PA, Dixon DA, Ekaykin A, Frezzotti M, Goodwin I, Isaksson E, Kaspari SD, Morgan VI, Oerter H, van Ommen TD, van der Veen CJ, and Wen JH. Insignificant change in antarctic snowfall since the international geophysical year, Science. d2006;313:827–31.
Nybakken L, Klanderud K, Totland O. Simulated environmental change has contrasting effects on defensive compound concentration in three alpine plant species. Arct Antarct Alp Res. 2008;40:709–15.
Ochyra R. The Moss Flora of King George Island. Cracow: Polish Academy of Sciences, W. Szafer Institute of Botany, Antarctica; 1998.
Ochyra R, Smith RIL, Bednarek-Ochyra H. The Illustrated Moss Flora of Antarctica. Cambridge: Cambridge University Press; 2008.
Olech, M. Lichens of King George Island, Antarctica. The Institute of Botany of the Jagiellonian University, Cracow, Poland. 2004.
Poortema K. On modelling overdispersion of counts. Statistica Neerlandica. 1999;53:5–20.
Reynolds DN. Gamete dispersal in Mnium ciliare. Bryologist. 1980;83:73–7.
Robinson SA, Wasley J, Tobin AK. Living on the edge - plants and global change in continental and maritime Antarctica. Global Change Biol. 2003;9:1681–717.
Royles J, Ogée J, Wingate L, Hodgson DA, Convey P, Griffiths H. Carbon isotope evidence for recent climate-related enhancement of CO2 assimilation and peat accumulation rates in Antarctica. Global Change Biol. 2012;18:3112–24.
Rydgren K, Cronberg N, Økland RH. Factors influencing reproductive success in the clonal moss, Hylocomium splendens. Oecol. 2006;147:445–54.
Schaeffer SM, Sharp E, Schimel JP, Welker JM. Soil-plant N processes in a High Arctic ecosystem, NW Greenland are altered by long-term experimental warming and higher rainfall. Global Change Biol. 2013;19:3529–39.
SAS Institute (2015) JMP for Windows. Release 12.0.1. Cary, N.C.
Selkirk PM. Vegetative reproduction and dispersal of Bryophyteson subantarctic Macquarie Island and in Antarctica. J Hatt Bot Lab. 1984;55:105–11.
Seppelt RD, Green TGA, Schwarz AMJ, Frost A. Extreme southern location for moss sporophytes in Antarctica. Antarct Sci. 1992;4:37–9.
Silva Wanderley H, Barbosa Justino F, Chuaco Sediyama G. Tendência da Temperatura e Precipitação na Península Antártica. Revista Brasileira de Meteorologia. 2016;31:114–21.
Smith RIL. Biological and environmental characteristics of three cosmopolitan mosses dominant in continental Antarctica. J Veg Sci. 1999;10:231–42.
Smith RIL, Convey P. Enhanced sexual reproduction in bryophytes at high latitudes in the maritime Antarctic. J Bryol. 2002;24:107–17.
Smith RIL. The bryophyte flora of geothermal habitats on Deception Island, Antarctica. J Hatt Bot Lab. 2005;97:233–48.
Stark LR. Widespread sporophyte abortion following summer rains in Mojave Desert populations of Grimmia orbicularis. Bryol. 2001;104:115–25.
Stark LR, Brinda JC, McLetchie DN. An experimental demonstration of the cost of sex and a potential resource limitation on reproduction in the moss Pterygoneurum (Pottiaceae). Am J Bot. 2009;96:1712–21.
Stark LR, Nichols II L, McLetchie DN, Bonine ML. Do the sexes of the desert moss Syntrichia caninervis differ in desiccation tolerance? A leaf regeneration assay. Int J Plant Sci. 2005;166:21–9.
Stark LR, Mishler BD, McLetchie DN. The cost of realized sexual reproduction: Assessing patterns of reproductive allocation and sporophyte abortion in a desert moss. Am J Bot. 2000;87:1599–608.
Stark LR, Stephenson AG. Reproductive biology of Entodon cladorrhizans (Bryopsida, Entodontaceae). II. Resource-limited reproduction and sporophyte abortion. Syst Botany. 1983;8:389–94.
Steig EJ, Schneider DP, Rutherford SD, Mann ME, Comiso JC, Shindell DT. Warming of the Antarctic ice-sheet surface since the 1957 international geophysical year. Nature. 2009;457:459–62.
Turner J, Colwell SR, Harangozo SA. Variability of precipitation over the coastal western Antarctic Peninsula from synoptic observations. J Geophys Res. 1997;102:13999–4007.
Turner J, Colwell SR, Marshall GJ, Lachlan-Cope TA, Carleton AM, Jones PD, Lagun V, Reid PA, Iagovkina S. Antarctic climate change during the last 50 years, Int J Climatol. 2005;25:279–94.
Turner J, Overland J. Contrasting climate change in the two polar regions. Polar Res. 2009;28:146–64.
Turner J, Bindschadler RA, Convey P, di Prisco G, Fahrbach E, Gutt J, Hodgson DA, Mayewski PA, Summerhayes CP. Antarctic Climate Change and the Environment, Scientific Committee on Antarctic Research. 2009.
Turner J, Barrand NE, Bracegirdle TJ, Convey P, Hodgson DA, Jarvis M, Jenkins A, Marshall G, Meredith MP, Roscoe H, Shanklin J, French J, Goosse H, Guglielmin M, Gutt J, Jacobs S, Kennicutt MC, Masson-Delmotte V, Mayewski P, Navarro F, Robinson S, Scambos T, Sparrow M, Summerhayes C, Speer K, Klepikov A. Antarctic climate change and the environment: an update. Polar Rec. 2014;50:237–59.
Une K, Yamaguchi T. Male plants of the Japanese species of Leucobryum hampe (Leucobryaceae, Musci). Hikobia. 2001;13:579–90.
Webb R. Reproductive behavior of mosses on Signy Island, South Orkney Islands. Brit Antarct Surv Bull. 1973;36:61–77.
Wyatt R, Anderson LE. Breeding systems in bryophytes. In: Dyer AF, Duckett JG, editors. The Experimental Biology of Bryophytes. London: Academic; 1984. p. 39–64.
Acknowledgments
We would like to thank the staff of Julio Escudero Scientific Station for the logistic facilities offered during the ECA (45th-46th Antarctic Scientific Expedition) of 2009 and 2010. Also, we are highly grateful for identification of Antarctic moss species by Dr. Juan Larraín and the field technical assistance of Gloria Gallegos-Haro. This study was done under the project INACH T0307 and FONDECYT 1120895. GA Torres-Mellado would like to thank the Chilean National Council for Science and Technology CONICYT for his Master’s Scholarship. SM Eppley would like to thank the US National Science Foundation (PLR1341742) for support. Angelica Casanova-Katny thanks the Vicerrectoría de Investigación, Desarrollo e Innovación of the Universidad Católica de Temuco. We thanks Dra. Marta María Caballero for the map of Fildes Peninsula. This study forms part of the integrated output of the Scientific Committee on Antarctic Research Biology Programme - State of the Antarctic Ecosystem- (AntEco). Thank you for useful comments of the anonymous reviewers of the final version of this paper.
Funding
In Chile, INACH T0307, FONDECYT 1120895 and the US National Science Foundation (PLR1341742).
Availability of data and materials
Not applicable.
Authors’ contributions
ACK carried out field experiments; GTM and ACK collected samples of mosses in Antarctica; GTM, ACK and SME analyzed and intrepreted the results. ACK and SME wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Casanova-Katny, A., Torres-Mellado, G.A. & Eppley, S.M. Reproductive output of mosses under experimental warming on Fildes Peninsula, King George Island, maritime Antarctica. Rev. Chil. de Hist. Nat. 89, 13 (2016). https://doi.org/10.1186/s40693-016-0061-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40693-016-0061-y