Abstract
Background
Fever during pregnancy is a relatively common and most often trivial event. However, under specific conditions, it could affect significantly fetal brain development. Few studies, with inconsistent results, investigated whether fever, regardless the pathogen, could represent a risk factor for neurodevelopmental disorders (NDD) in the offspring. We aimed to explore further this question by performing a systematic review and meta-analysis.
Methods
Peer-reviewed studies exploring the occurrence of NDD in offspring after a fetal exposure to maternal fever were included. We specifically considered the impact of fever severity and duration, taking into consideration some confounding variables such as the use of antipyretic during pregnancy, the trimester in which the fever arose, the maternal age or smoking at time of gestation. MEDLINE, EMBASE, PsycINFO, Cochrane and Web of Science were searched without language restriction. PRISMA recommendations were followed. Odds ratio (OR) were pooled using random-effects meta-analysis. Heterogeneity in effect size across studies was studied using random-effects meta-regression analysis. (PROSPERO CRD42020182801).
Results
We finally considered ten studies gathering a total of 10,304 children with NDD. Among them, 1394 were exposed to fever during pregnancy. The selected studies were divided into 5 case–control studies and 5 cohort studies. Maternal exposure to fever during pregnancy increased the risk of NDD in offspring with an OR of 1.24 [95% CI: 1.12–1.38]. Secondary analysis revealed an increased risk for NDD when fever occurred during the first trimester of gestation [OR 1.13–95% CI: 1.02–1.26].
Limitations
We excluded studies that considered infections with no evidence of fever. Another potential limitation may be the possible heterogeneity between study designs (cohorts and case–control).
Conclusion
Additional evidence supported the association between fever during pregnancy and increased risk for NDD in offspring. Careful monitoring should be considered for children born from mothers with a febrile episode during pregnancy (specifically during the first trimester).
Similar content being viewed by others
Background
Neurodevelopmental disorders (NDD) are a heterogeneous group of illnesses that begin during a child’s early brain development [1] and range from specific learning difficulties to severe cognitive impairments [1]. The determinism of NDD remains unclear but depends on the interaction of genetic vulnerability and environmental insults [2, 3]. Twin studies upheld this gene-environment interaction model reporting up to 90% concordance rate in monozygotic twins and 50% in dizygotic [1, 4]. Some of the brain Magnetic Resonance Imaging (MRI) abnormalities reported in children with NDD might suggest a prenatal pathogenesis [5, 6]. Therefore, many authors investigated the role of environmental stressors occurring during the pregnancy, with a specific focus on infections or fever [7].
Fever during pregnancy is common, with approximately 20% of women reporting at least one febrile episode [8, 9]. Despite being a relatively benign condition, fever was associated with several neonatal affections and adverse health affections in offspring, leading sometimes to a significant referral to intensive care [10]. Added to this, evidence from animal studies supplemented the suspicions of fever-induced neurotoxicity, independently from the direct impact of infectious processes [11, 12]. The effect of maternal fever during pregnancy may also explain by itself the increased prevalence of NDD observed in the offspring [3, 13, 14]. This association was suggested to occur secondarily to maternal immune activation (MIA), rather than the direct effect of the pathogen on the fetal brain [14, 15]. In animal studies, MIA during gestation affected the brain development and resulted in behavioral abnormalities, mimicking those from NDD in humans [3, 16, 17]. MIA in rodents and monkeys resulted in socio-communication deficit and stereotyped behaviors in pups similar to the cardinal symptoms of autism spectrum disorder (ASD) in children [18]. Consequently, theories have been raised concerning the detrimental impact of maternal inflammatory balance disruption on the fetal brain development [3]. Maternal pro-inflammatory cytokine interleukin-6 (Il-6) elevation or interleukin-10 (Il-10) decrease, both observed during MIA, might have a key role in yielding abnormal neurodevelopment in offspring [17, 19]. While current evidence did not bring out a direct causal link, genetic susceptibility probably intervened also in the subsequent NDD-related symptoms [3].
However, the literature on fever during pregnancy remains vague on factors modulating the risk of developing a NDD in offspring. For example, the period of pregnancy during which fetal vulnerability is greatest is poorly known. Based on animal models of MIA and taking into account the development of the fetal brain in utero, we assumed that the end of the first trimester and the beginning of the second trimester of pregnancy could represent the period of greatest risk [6, 20,21,22]. Nevertheless, the neurogenesis begins in the early weeks of pregnancy and the first trimester may also represent an important time window for the fetal risk. This period corresponds to an active period of division and migration of neurons [23]. The use of antipyretic medications during gestation might also be NDD risk factor for offspring, by counteracting the protective effect of anti-inflammatory cytokines. For example, some studies suggested that acetaminophen use during pregnancy increased the risk of Attention-Deficit/Hyperactivity Disorder (ADHD) [24, 25]. Contrariwise, others showed that antipyretics would have a protective effect on NDD emergence in offspring [21, 26,27,28].
Herein, we present the results of a systematic review and meta-analysis of the literature which explored the association between prenatal exposure to fever, no matter the cause, and NDD outcomes in the offspring.
Methods
Search strategy and selection criteria
We performed a systematic review and meta-analysis following the PRISMA recommendations (Preferred Reporting Items for Systematic review and Meta-Analysis) [29]. Studies were included in our systematic review if they met the following criteria: (1) reported the occurrence of any type of NDD that corresponds to DSM-5 criteria; (2) mentioned the history of fever during pregnancy; (3) included a control group that was not diagnosed with any type of NDD (other non-NDD psychiatric controls were not eligible); (4) were with sufficient sample size to allow computation of an odds ratio (OR). Exclusion criteria were: (1) studies assessing only symptoms of NDD but without formal diagnosis; (2) children screened before age 36 months (no formal diagnosis); (3) the sample overlapping (and being smaller) with another eligible study i.e. if studies used the same cohort, we only included the publication reporting the largest number of participants to avoid duplication of data; (4) animal studies.
We searched MEDLINE (1946 to July, 2020), EMBASE (1974 to July, 2020), PsycINFO (1806 to July, 2020), Cochrane Central Register of Controlled Trials (CENTRAL; from inception to July, 2020), and Web of Science Core Collection (1900 to July, 2020) without any restrictions on language, ethnic origins, date or article type. Both cohort and case–control studies were included. The research was based on a combination of medical subject heading (Table 1). We also searched the references in the included studies for any potential pertinent study not detected in the search of databases [29].
The eligibility process was conducted in two separate stages: (1) two researchers (SA and PE) independently screened all non-duplicated references initially retrieved as potentially pertinent and excluded those clearly not pertinent based on title or abstract. A final list was agreed with discrepancies resolved by consensus between the two authors. When consensus was not reached, a third, senior researcher (RD) acted as arbitrator; (2) the full-text version of the articles passing stage 1 screening was downloaded and assessed for eligibility by the two researchers, independently. Discrepancies were resolved by consensus between the two researchers and, if needed, the third senior researcher also acted as arbitrator. When required, corresponding authors were contacted to clarify study eligibility.
The protocol for the present systematic review/meta-analysis was pre-registered on the international Prospective Register of Systematic Reviews PROSPERO (protocol number: PROSPERO CRD42020182801).
Data analysis and outcomes
The main outcome measure was the OR expressing the association between fever in mothers during pregnancy and NDD in the offspring. To further estimate the risk of NDD in the offspring, we performed secondary outcome analysis by taking into account: (1) the types of NDD and the criteria used for their definition; (2) the fever related information regarding the trimester in which it occurred, the prescription of antipyretic medication, and its duration (in days) and the highest fever reached during pregnancy; (3) the offspring’s related main confounding variables including age, sex, week gestational age (WGA) at delivery; and (4) the mothers’ related information: age at conception, smoking during pregnancy and personal history of psychiatric disorders. In addition to these variables, we extracted some information regarding authors, year of publication, country in which the study was conducted.
To assess the quality of studies included in the meta-analysis, SA and PE used a modified version of the Newcastle Ottawa Scale (NOS) [30, 31]. Briefly, the NOS provides assessment criteria for case–control, cross-sectional and cohort studies. Three methodological domains were assessed: the selection criteria, the comparability and the measurement of outcome/exposure. Scoring criteria were amended such that the maximum score available for each study was 8 (Tables 2 and 3). Studies were of high quality if the NOS score was strictly > 4.
We used a random-effect meta-analysis model. Heterogeneity was assessed using the I2 statistic and Tau2. The I2 score represents the percentage of variation across studies due to heterogeneity, and ranges from zero to 100%; higher values indicate greater heterogeneity across studies [32]. Tau2 estimates the between study variance in a random-effect meta-analysis. A Tau2 value greater than 1 suggests the presence of substantial heterogeneity [33,34,35]. The statistical analysis was performed using the R package “meta” for unadjusted OR and “metaphor” for adjusted OR (log-transformed). Meta-analysis of OR was done only when three or more studies were available. To assess publication bias, we used funnel plots. We also performed metaregression on the following confounding factors: impact of fever duration, antipyretic medication, and maternal characteristics. Metaregression aims to examine the impact of the confounding factors on the study. Additional statistical analyses were carried out to stratify by NDD type and by study design, followed by cross stratification.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Literature search and selection
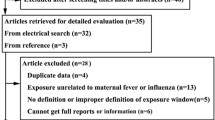
An initial pool of 60 articles was identified through database searching (MEDLINE, EMBASE, PsyINFO, Cochrane Central Register of Controlled Trials and Web of Science Core Collection). In all, 3 animal experiments, 13 reviews and 24 irrelevant studies were excluded. The eligibility of 20 studies were assessed by full-text review. Of the latter, 10 studies were subsequently excluded [24, 25, 36,37,38,39,40,41,42,43]: five had other primary exposures (acetaminophen use, infection without information about fever, premature birth), three lacked data on fever, one was without a control group, and one assessed the impact on academic performance instead of the occurrence of NDD (Table 4). Ten remaining articles [6, 14, 21, 22, 27, 44,45,46,47,48] met the eligibility criteria for inclusion (Fig. 1).
Characteristics of the included studies
The selected studies were published between 2012 and 2019. Five were case–control and five were cohort studies, with a total of 416,446 participants (cohort studies: 411,151; case–control studies: 5295). Among them, 81,124 women were with fever during pregnancy (cohort studies: 80,369; case–control studies: 755). Finally, 10,304 of the offspring were diagnosed with a NDD (cohort studies: 7620; case–control studies: 2684). Among them, 3224 were with a diagnosis of ASD (cohort studies: 1559; case–control studies: 1665), 5156 with a diagnosis of ADHD (only in cohort studies), 905 with a diagnosis of Developmental Coordination Disorder (DCD) (only in cohort studies), and 1019 with a diagnosis of Developmental Delay (DD) (only in case control studies). It is important to note that the cohort studies are from two study populations, namely the Danish National Birth Cohort (three studies) [6, 14, 47] and the Norwegian Mother and Child Cohort Study (two studies) [21, 48]. Characteristics of the selected studies were reported in Table 5.
Maternal exposure to fever during pregnancy and risk of NDD in offspring
A significant association was found between fever during pregnancy and the risk of NDD in offspring (p-value < 0.0001), with a pooled odds ratio (OR) of 1.24 [95% confidence interval (CI) 1.12–1.38] (Fig. 2). There was however evidence of heterogeneity [Tau2 = 0.016, degrees of freedom (df) = 11, p < 0.001, I2 = 61.02] and we observed a funnel plot asymmetry (Additional file 1: Supplemental figures). The group disparity appeared as the result of small sample sizes in some of the selected studies.
Stratification analyses
Additional statistical analyses were carried out to stratify by NDD type. There was a positive association for ASD (p = 0.03), with an OR of 1.15 [95% CI 1.01–1.31], and a low evidence of heterogeneity [Tau2 = 0.0076, df = 6, p = 0.24, I2 = 24.9%]. We also observed a positive association for DD (p = 0.03) with an OR of 1.23 [95% CI 1.07–1.41] and low heterogeneity [Tau2 = 0.0019, df = 2, p = 0.33, I2 = 9.7%]. There was no possibility to calculate the OR for ADHD, with only two studies, nor for DCD, with only one study.
Stratification by type of study design were also carried out. We found a positive association (p = 0.01) for case control studies taken alone with an OR of 1.19 [95% CI 1.03–1.38], with no evidence of heterogeneity [Tau2 = 0, df = 6, p = 0.46, I2 = 0%]. There was also a positive association for cohort studies (p = 0.004) with an OR of 1.20 [95% CI 1.06–1.36], with high evidence of heterogeneity [Tau2 = 0.0152, df = 4, p = 0.0015, I2 = 77.3%].
We carried out cross stratification between study design and specific NDD type. Only ASD in case control studies were taken into account due to the lack of data. We found a positive association (p = 0.02) with an OR of 1.24 [95% CI 1.02–1.49], without heterogeneity [Tau2 = 0, df = 4, p = 0.41, I2 = 0%].
Secondary analysis and risk of NDD in offspring
An analysis of fever characteristics was conducted to explore sources of heterogeneity in the link between maternal exposure to fever during pregnancy and NDD in the offspring. Six studies [14, 21, 22, 46,47,48] investigated the effect of maternal fever according to the trimester during which it occurred. Positive association (p = 0.024) was noted during the first trimester of pregnancy [OR 1.13; 95% CI 1.02–1.26], without evidence of heterogeneity [Tau2 = 0, df = 6, p = 0.365, I2 = 0] (Fig. 3). We however did not observe any association between the risk of NDD and the fever occurrence during the second or the third trimesters of pregnancy [OR 1.22 (95% CI 0.92–1.61) and OR 1.07 (95% CI 0.93–1.23) respectively] (Figs. 4 and 5). There was evidence of heterogeneity during the second trimester of pregnancy [Tau2 = 0.096, df = 6, p = 0.0008, I2 = 82.34], but not during the third trimester [Tau2 = 0, df = 5, p = 0.7741, I2 = 0]. Funnel plots suggested a potential publication bias for the first and second trimesters, but not for the third one (see Additional file 1: supplemental figures).
Three studies [14, 21, 47] explored the association between fever duration and the risk of NDD in the offspring. There was a trend of an increased risk (p = 0.072) when fever exceeded 3 days in the mother [OR 1.24 (95% CI 0.98–1.57)] compared to a shorter duration [OR 1.12 (95% CI 0.97–1.29), p = 0.1188]. We observed heterogeneity values for a duration longer than 3 days [Tau2 = 0.019, df = 2, p = 0.043, I2 = 45.91], but not when the studies reported a fever duration shorter than 3 days [Tau2 = 0.0064, df = 2, p = 0.197, I2 = 39.70]. Funnel plots showed no publication bias (Figs. 6 and 7).
Additional secondary analysis suggested that the use of anti-pyretic medication in the context of fever during pregnancy [6, 14, 21, 27, 44] did not significantly affect the risk of NDD in the offspring (p = 0.642). There was however evidence for heterogeneity in the studies [Tau2 = 0.2462, df = 4, p < 0.0001, I2 = 88.24], even in the absence of publication bias (Fig. 8). The other variables we initially selected for the secondary analysis, were not taken into consideration in our results because either they were explored by fewer than three studies, or the metric used to quantify them were not similar among studies.
Meta-regression: impact of fever duration, antipyretic medication, and maternal characteristics on NDD risk
To further examine the impact of fever duration (k = 3) on the risk of NDD in offspring, we performed a meta-regression. The analysis showed a non-significant effect of prolonged duration (p = 0.313), with evidence of heterogeneity [Tau2 = 0.0260, df = 1, p = 0.0164, I2 = 82.64]. Similarly, the meta-regression model (k = 7) for the use of antipyretic medications confirmed the previous findings with no significant association (p = 0.264) and evidence of heterogeneity [Tau2 = 0.0097, df = 5, p = 0.0351, I2 = 54.28] (Fig. 9). We finally explored the effect of maternal age at birth, maternal smoking during pregnancy, and the gestational age at birth. The meta-regression for a maternal age greater than 35 years old (k = 4) was a significant covariate (p = 0.014), with no heterogeneity [Tau2 = 0, df = 2, p = 0.5142, I2 = 0] (Fig. 10). There was no statistically significant association with gestational age at birth over 37 WGA (k = 3, p = 0.1296) with evidence of heterogeneity [Tau2 = 0.0005, df = 1, p = 0.2991, I2 = 89.90]. Similarly, no association was observed with maternal smoking during pregnancy (k = 4, p = 0.6337) with evidence of heterogeneity [Tau2 = 0.0132, df = 2, p = 0.009, I2 = 75.29].
Discussion
Our meta-analysis aimed to clarify the relationship between prenatal exposure to fever and neurodevelopmental outcomes in the offspring. Based on a meta-analysis gathering a total of 416,446 pregnant women including 81,124 children who were exposed to maternal fever during pregnancy, we reported an increased risk of NDD in the offspring with an odds-ratio (OR) of 1.24 [95% CI: 1.12–1.38], specifically during the first trimester of pregnancy [OR 1.13; 95% CI 1.02–1.26]. This risk was particularly relevant for ASD (OR 1.15, 95% CI: 1.01–1.31) and DD (OR 1.23, 95% CI: 1.07–1.41).
The increased risk of NDD may directly rise from the increased levels of cytokines and imbalance in T cell populations associated with fever, occurring in a critical window of the fetal brain development [49, 50]. This acute maternal immune dysregulation may affect the in utero environmental homeostasis, required for fetal brain development [49]. Animal models upheld this mechanism, showing a significant deviation in fetal neuron divisions and migrations [17, 51]. Despite infections being the most common route to MIA, fever by itself induced the release of non-specific cytokines, such as Il-6, in pregnant women [14, 52]. It is unclear how peripheral maternal immune signals impacted the brain development [52] but animal studies found that systemic maternal Il-6 secretion was sufficient to induce ASD-like phenotype in the offspring [17]. Added to this, recent studies also suggested that a maternal exposure to stress might induce a downregulation in RNA Binding Motif protein 3 (RBM3) in the developing brain [53]. While RBM3 neuroprotective properties are applied in induced hypothermia, as well as other life-threatening conditions that require cell protection, the mechanisms regulating its expression remained insufficiently covered [53].
Interestingly, we found a significant association between fever during the first trimester of pregnancy and the risk of NDD, in contrast with the last trimester of gestation. Even though the underlying neurobiological hypothesis was not clearly defined, the first two trimesters, specifically between 13 and 27 WGA, entailed an immune profile for appropriate fetal brain growth, characterized by a secretion of Transforming Growth Factor β (TGF-β). The association of fever with increased levels of both Il-6 and TGF-β may directly affect maternal immune cell homeostasis leading to defects in the fetal brain architecture development [20, 54].
Surprisingly, we did not observe that fever duration or anti-pyretic medications during pregnancy influenced the neurodevelopmental risk [6, 14, 21, 27, 44]. These results based on subsamples of subjects may be however unpowered to detect a significant effect on NDD risk. Additionally, we combined antipyretic medications (e.g. acetaminophen, ibuprofen) and antibiotic drugs (only one single study was solely with antibiotics [14]) to explore the whole effect of drugs administrated in the context of fever during pregnancy. A recent meta-analysis found a positive association between acetaminophen and ADHD, with an increased OR of 1.25 (95% CI [1.17, 1.34]) [55]. It is also important to note that recent research suggested that acetaminophen use during third trimester (not only for fever) was associated with ADHD, and ASD onset in the offspring [56].
The meta-regression we ran on maternal confounding factors reported a significant cross-effect for maternal age over 35 years old. This was consistent with previous findings that provided evidence for advanced maternal age as a substantial risk factor for NDD, specifically ASD [7]. We were however unable to detect the cumulative effect of maternal smoking and fever during pregnancy on the risk of NDD. While it was suggested that oxidative stress would trigger the detrimental effects of maternal smoking on fetal brain development, it seemed that maternal fever might follow a distinct pathway [57]. Therefore, these two risk factors might not be closely connected, thus explaining the weak interaction we observed in our results.
Emerging infections drew attention to the impact of fever on the offspring of infected pregnant women [58]. The recent global outbreak of the SARS-CoV-2 raised concerns over the impact of the virus on brain development [58,59,60]. With still insufficient information on the vertical transmission [61], we need to carefully monitor the developmental trajectory of babies from pregnant women infected by the SARS-CoV-2, specifically in mothers displaying a seroconversion [59].
Limitations
Although our results were congruent with previous findings in the literature, our meta-analysis should be considered in light of its limitations. First, the inclusion process excluded studies that considered exposure to infections without information on fever. As suggested previously, distinguishing fever outcomes from those of infections might be difficult, giving the fact that both conditions could enhance MIA [14, 26]. Fever as a symptom might not be always present, letting an underlying infection go undetected. Conversely, non-infectious fever is also common during pregnancy but with significant effect on maternal immune regulation [51]. Second, the methodological heterogeneity between cohorts and case–control studies we assumed in our study, may be considered as a publication bias. However, stratification analysis did not support this hypothesis. Similarly, in the subgroup analysis, confounding analysis was limited by the absence of overlapping data between the different studies, especially concerning etiology and administered treatment for fever. Third, we combined the effect of symptomatic treatment for fever and the use of antibiotics under the denomination of antipyretic treatment. While the use of both types of treatment has either a direct or an indirect effect on decreasing the fever, they have different mechanisms that could be linked to the risk of NDD in offspring [14]. Finally, the majority of our population comes from two sources, namely the Danish and the Norwegian cohorts; this could limit generalizability and possibly bias our results. Further studies should take into consideration all that preceded in order to optimize their results.
Conclusion
In conclusion, our results provided further evidence for an association between maternal fever during pregnancy and NDD risk in the offspring. In line with our results, and in the absence of consensus, it is wise to treat maternal fever accordingly, in order to prevent the previously described subsequent neuroinflammation [28]. In the specific context of SARS-CoV-2 pandemic, careful monitoring for children born from mothers with a febrile episode during pregnancy, specifically during the first trimester and with a seroconversion should be considered [59].
Availability of data and materials
Not applicable.
References
Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub; 2013.
DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, et al. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26(26):6897–906.
Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17(7):389–94.
Coe BP, Girirajan S, Eichler EE. The genetic variability and commonality of neurodevelopmental disease. In: American Journal of Medical Genetics Part C: seminars in medical genetics. Wiley Online Library; 2012. p. 118–29.
Newschaffer CJ, Fallin D, Lee NL. Heritable and nonheritable risk factors for autism spectrum disorders. Epidemiol Rev. 2002;24(2):137–53.
Werenberg Dreier J, Nybo Andersen A-M, Hvolby A, Garne E, Kragh Andersen P, Berg-Beckhoff G. Fever and infections in pregnancy and risk of attention deficit/hyperactivity disorder in the offspring. J Child Psychol Psychiatry. 2016;57(4):540–8.
Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry. 2009;195(1):7–14.
Andersen A-MN, Vastrup P, Wohlfahrt J, Andersen PK, Olsen J, Melbye M. Fever in pregnancy and risk of fetal death: a cohort study. Lancet. 2002;360(9345):1552–6.
Collier SA, Rasmussen SA, Feldkamp ML, Honein MA. Prevalence of self-reported infection during pregnancy among control mothers in the National Birth Defects Prevention Study. Birt Defects Res A Clin Mol Teratol. 2009;85(3):193–201.
Lieberman E, Lang J, Richardson DK, Frigoletto FD, Heffner LJ, Cohen A. Intrapartum maternal fever and neonatal outcome. Pediatrics. 2000;105(1):8–13.
Edwards MJ. Hyperthermia as a teratogen: a review of experimental studies and their clinical significance. Teratog Carcinog Mutagen. 1986;6(6):563–82.
Graham JM Jr, Edwards MJ, Edwards MJ. Teratogen update: gestational effects of maternal hyperthermia due to febrile illnesses and resultant patterns of defects in humans. Teratology. 1998;58(5):209–21.
Brown AS, Susser ES. In utero infection and adult schizophrenia. Ment Retard Dev Disabil Res Rev. 2002;8(1):51–7.
Atladóttir HÓ, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics. 2012;130(6):e1447-1454.
Patterson PH. Maternal influenza infection leads to neuropathology and behavioral abnormalities in adult offspring. In: Neuropsychopharmacology. Nature Publishing Group Macmillan Building, 4 Crinan St, London N1 9XW, England; 2005. p. S9–S9.
Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23(1):297–302.
Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27(40):10695–702.
Malkova N. Maternal immune activation causes a deficit in social and communicative behavior in male mouse offspring. Program No. 561.29. Neurosci Mtg Plan San Diego Soc Neurosci. 2010;
Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry. 2008;13(2):208–21.
Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351(6276):933–9.
Hornig M, Bresnahan MA, Che X, Schultz AF, Ukaigwe JE, Eddy ML, et al. Prenatal fever and autism risk. Mol Psychiatry. 2018;23(3):759–66.
Croen LA, Qian Y, Ashwood P, Zerbo O, Schendel D, Pinto-Martin J, et al. Infection and fever in pregnancy and autism spectrum disorders: findings from the study to explore early development. Autism Res Off J Int Soc Autism Res. 2019;12(10):1551–61.
Dombrowski SC, Martin RP, Huttunen MO. Association between maternal fever and psychological/behavior outcomes: a hypothesis. Birt Defects Res A Clin Mol Teratol. 2003;67(11):905–10.
Liew Z, Ritz B, Rebordosa C, Lee P-C, Olsen J. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr. 2014;168(4):313–20.
Ystrom E, Gustavson K, Brandlistuen RE, Knudsen GP, Magnus P, Susser E, et al. Prenatal exposure to acetaminophen and risk of ADHD. Pediatrics. 2017;140(5).
Dreier JW, Andersen A-MN, Berg-Beckhoff G. Systematic review and meta-analyses: fever in pregnancy and health impacts in the offspring. Pediatrics. 2014;133(3):e674–88.
Zerbo O, Iosif A-M, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord. 2013;43(1):25–33.
Pinto B, Morelli G, Rastogi M, Savardi A, Fumagalli A, Petretto A, et al. Rescuing Over-activated Microglia Restores Cognitive Performance in Juvenile Animals of the Dp(16) Mouse Model of Down Syndrome. Neuron [Internet]. 2020 Oct 6 [cited 2020 Oct 12]. https://www.cell.com/neuron/abstract/S0896-6273(20)30710-8
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 21;6(7):e1000097.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Cullen AE, Holmes S, Pollak TA, Blackman G, Joyce DW, Kempton MJ, et al. Associations between non-neurological autoimmune disorders and psychosis: a meta-analysis. Biol Psychiatry. 2019;85(1):35–48.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Borenstein M, Higgins JPT, Hedges LV, Rothstein HR. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8(1):5–18.
Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. New York: Wiley; 2008.
Suijkerbuijk YB, Schaafsma FG, Mechelen JC van, Ojajärvi A, Corbière M, Anema JR. Interventions for obtaining and maintaining employment in adults with severe mental illness, a network meta‐analysis. Cochrane Database Syst Rev [Internet]. 2017 [cited 2020 May 21];(9). https://www.cochranelibrary.com/cdsr/doi/https://doi.org/10.1002/14651858.CD011867.pub2/full
Al-Dawood K, Albar A. Risk factors of mental retardation in children attending an educationally subnormal/mental school in Dammam. Saudi Arabia Ann Saudi Med. 1993;13(4):355–9.
Dreier JW, Berg-Beckhoff G, Andersen PK, Andersen A-MN. Prenatal exposure to fever and infections and academic performance: a multilevel analysis. Am J Epidemiol. 2017;186(1):29–37.
Joseph RM, Korzeniewski SJ, Allred EN, O’Shea TM, Heeren T, Frazier JA, et al. Extremely low gestational age and very low birthweight for gestational age are risk factors for autism spectrum disorder in a large cohort study of 10-year-old children born at 23–27 weeks’ gestation. Am J Obstet Gynecol. 2017;216(3):304.e1-304.e16.
Liew Z, Ritz B, Virk J, Olsen J. Maternal use of acetaminophen during pregnancy and risk of autism spectrum disorders in childhood: a Danish national birth cohort study. Autism Res Off J Int Soc Autism Res. 2016;9(9):951–8.
Mattson SN, Jones KL, Gramling LJ, Schonfeld AM, Riley EP, Harris JA, et al. Neurodevelopmental follow-up of children of women infected with varicella during pregnancy: a prospective study. Pediatr Infect Dis J. 2003;22(9):819–23.
Mazina V, Gerdts J, Trinh S, Ankenman K, Ward T, Dennis MY, et al. Epigenetics of autism-related impairment: copy number variation and maternal infection. J Dev Behav Pediatr JDBP. 2015;36(2):61–7.
Pineda DA, Puerta IC, Merchán V, Arango CP, Galvis AY, Velásquez B, et al. Perinatal factors associated with attention deficit/hyperactivity diagnosis in Colombian Paisa children. Rev Neurol. 2003;36(7):609–13.
Zerbo O, Qian Y, Yoshida C, Fireman BH, Klein NP, Croen LA. Association between influenza infection and vaccination during pregnancy and risk of autism spectrum disorder. JAMA Pediatr. 2017;171(1):e163609.
Saunders A, Woodland J, Gander S. A comparison of prenatal exposures in children with and without a diagnosis of autism spectrum disorder. Cureus. 2019;11(7):e5223.
Christian MA, Samms-Vaughan M, Lee M, Bressler J, Hessabi M, Grove ML, et al. Maternal exposures associated with autism spectrum disorder in Jamaican children. J Autism Dev Disord. 2018;48(8):2766–78.
Brucato M, Ladd-Acosta C, Li M, Caruso D, Hong X, Kaczaniuk J, et al. Prenatal exposure to fever is associated with autism spectrum disorder in the boston birth cohort. Autism Res Off J Int Soc Autism Res. 2017;10(11):1878–90.
Holst C, Jørgensen SE, Wohlfahrt J, Nybo Andersen A-M, Melbye M. Fever during pregnancy and motor development in children: a study within the Danish National Birth Cohort. Dev Med Child Neurol. 2015;57(8):725–32.
Gustavson K, Ask H, Ystrom E, Stoltenberg C, Lipkin WI, Surén P, et al. Maternal fever during pregnancy and offspring attention deficit hyperactivity disorder. Sci Rep. 2019;9(1):9519.
Patel S, Cooper MN, Jones H, Whitehouse AJO, Dale RC, Guastella AJ. Maternal immune-related conditions during pregnancy may be a risk factor for neuropsychiatric problems in offspring throughout childhood and adolescence. Psychol Med. 2020;1:1–11.
Meltzer A, Van de Water J. The role of the immune system in autism spectrum disorder. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2017;42(1):284–98.
Pancaro C, Boulanger-Bertolus J, Segal S, Watson CJ, St Charles I, Mashour GA, et al. Maternal noninfectious fever enhances cell proliferation and microglial activation in the neonatal rat dentate gyrus. Anesth Analg. 2019;128(6):1190–8.
Blomqvist A, Engblom D. Neural mechanisms of inflammation-induced fever. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry. 2018;24(4):381–99.
Zhu X, Bührer C, Wellmann S. Cold-inducible proteins CIRP and RBM3, a unique couple with activities far beyond the cold. Cell Mol Life Sci. 2016;73(20):3839–59.
Ito JT, Cervilha DA de B, Lourenço JD, Gonçalves NG, Volpini RA, Caldini EG, et al. Th17/Treg imbalance in COPD progression: a temporal analysis using a CS-induced model. PloS One. 2019;14(1):e0209351.
Gou X, Wang Y, Tang Y, Qu Y, Tang J, Shi J, et al. Association of maternal prenatal acetaminophen use with the risk of attention deficit/hyperactivity disorder in offspring: a meta-analysis. Aust N Z J Psychiatry. 2019;53(3):195–206.
Ji Y, Azuine RE, Zhang Y, Hou W, Hong X, Wang G, et al. Association of cord plasma biomarkers of in utero acetaminophen exposure with risk of attention-deficit/hyperactivity disorder and autism spectrum disorder in childhood. JAMA Psychiat. 2020;77(2):180–9.
Kalkbrenner AE, Meier SM, Madley-Dowd P, Ladd-Acosta C, Fallin MD, Parner E, et al. Familial confounding of the association between maternal smoking in pregnancy and autism spectrum disorder in offspring. Autism Res Off J Int Soc Autism Res. 2020;13(1):134–44.
Rasmussen SA, Hayes EB. Public health approach to emerging infections among pregnant women. Am J Public Health. 2005;95(11):1942–4.
Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. Coronavirus Disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222(5):415–26.
World Health Organization. Coronavirus disease (COVID-19) outbreak [Internet]. https://www.who.int/emergencies/diseases/novelcoronavirus-2019
Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11(1):3572.
Acknowledgements
The authors would like to thank the following authors who kindly provided additional unpublished information/data: A. Saunders and colleagues, Department of Internal Medicine, Saint John Regional Hospital, Saint John, Canada; H. Ó. Atladóttir and colleagues, Department of Public Health, Section of Epidemiology, University of Aarhus, Aarhus, Denmark; K. Gustavson and colleagues, Norwegian Institute of Public Health, University of Oslo, Oslo, Norway.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SA and PE did the bibliography, collected data, carried out the initial analyses, and drafted the initial manuscript. HP carried out the statistical analysis, and reviewed and revised the manuscript. MR, DK and PG gave input for the draft. RD supervised the study and critically reviewed the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Full data extracted and funnel plots.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Antoun, S., Ellul, P., Peyre, H. et al. Fever during pregnancy as a risk factor for neurodevelopmental disorders: results from a systematic review and meta-analysis. Molecular Autism 12, 60 (2021). https://doi.org/10.1186/s13229-021-00464-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13229-021-00464-4