Abstract
Background
Nur77, a key member of the NR4A receptor subfamily, is involved in the regulation of inflammation and immunity. However, the in vivo regulatory roles of Nur77 in sepsis and the mechanisms involved remains largely elusive. In this study, we used Nur77-deficient (Nur77−/−) mice and investigated the function of Nur77 in sepsis.
Findings
Compared to wild-type (Nur77+/+) mice, Nur77−/− mice are more susceptible to LPS-induced sepsis and acute liver inflammation. Mechanistically, we observed that Nur77 can interact with TRAF6, a crucial adaptor molecule in the Toll-like receptor-interleukin 1 receptor (TLR-IL-1R) signalling pathway, in in vivo mouse model of sepsis. The interaction may affect TRAF6 auto-ubiquitination, thereby inhibiting NF-κB activation and pro-inflammatory cytokines production.
Conclusions
These in vivo observations reveals an important protective role for Nur77 in LPS-induced sepsis through its regulation to TRAF6 signalling, and highlights the potential clinical application of Nur77 as a molecular target in prevention and/or treatment of sepsis.
Similar content being viewed by others
Introduction
Orphan nuclear receptor Nur77 (also called TR3, NGFI-B, or NR4A1) is a member of the NR4A family of nuclear receptors. Similar to other nuclear receptors, Nur77 consists of an N-terminal transactivation domain, a central DNA binding domain and a C-terminal ligand binding domain, and can act in the nucleus as a ligand-independent and constitutively active transcription factor by binding to its DNA response elements as monomers [1], homodimers [2] or heterodimers with retinoid X receptor [2]. Unlike other nuclear receptors, Nur77 and other members of the subfamily are classified as early response genes whose expression is induced by a diverse range of extracellular stimuli including a wide array of cytokines and growth factors [3]. Consistently, accumulating studies indicate that Nur77 is implicated in the control of inflammatory diseases including atherosclerosis [4], arthritis [5], inflammatory bowel disease (IBD) [6] and cancer [7]. Nur77 is aberrantly expressed in atherosclerotic lesions [8], and cancer [7]. Nur77 may act to mediate pro-inflammatory signalling by increasing the expression of NF-κB-activating kinase, IKKi [9] to attenuate cytokine signalling. However, recent in vivo studies have shown that Nur77 is protective against the development of atherosclerosis by regulating the polarization of macrophages and subsequently inhibits inflammatory responses [10], indicating Nur77 may mediate anti-inflammatory signalling. Despite all of these efforts, however, the in vivo roles and underlying mechanism of Nur77 in sepsis are unclear.
Tumor necrosis factor receptor associated factor 6 (TRAF6), a member of TRAF family, is a common signalling mediator for the TLR-IL-1R superfamily [11]. TRAF6-deficient mice have defects in TLR-IL-1R-initiated inflammatory signalling [12]. TRAF6 is reported to possess an E3 ubiquitin ligase and undergoes lysine 63 (K63)-linked auto-ubiquitination [13]. The modification, in contrast to K48-linked polyubiquitin conjugation, is not associated with proteasomal degradation but instead facilitates signal transduction and protein trafficking [14, 15]. The regulatory involvement of various molecules in TRAF6-mediated TLR-IL-1R signalling has been confirmed. β-arrestin act as negative regulators to prevent ubiquitination of TRAF6 through direct interaction, which effectively blocks excessive inflammatory responses [16]. In contrast, the formation of TRAF6-HSP27 complex promotes TRAF6 ubiquitination and enhances activation of NF-κB signalling triggered by IL-1β [17].
Here, we investigate the in vivo function of Nur77 in sepsis and sepsis-associated liver injury. Our work indicates that Nur77 deficiency in mice increased their susceptibility to LPS-induced sepsis and acute liver injury, and reveal a critical mechanism wherein Nur77 interacts with TRAF6 and regulate its auto-ubiquitination in in vivo mouse model of sepsis.
Materials and methods
Mice
Nur77-knockout mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were maintained in a pathogen-free environment as recently described [6]. All animal experiments were performed in accordance with the regulations and guidelines of the Animal Care and Use Committee of Soochow University.
Sepsis model
The age- and sex-matched Nur77+/+ versus Nur77−/− mice were injected with LPS (20 mg/kg, ip). Then, 2 h later, hepatic Tnf, Il6, and Il12b mRNA was measured by RT-PCR and Real-time PCR. TNFα and IL-6 in blood were measured with ELISA. To induce endotoxic shock, mice were injected with LPS (20 mg/kg, ip) and were monitored for survival for the ensuing 72 h.
Acute liver injury model
Nur77+/+ and Nur77−/− mice used for the model were 8–10 weeks of age and were matched for age and sex. Mice were co-injected with LPS (5 μg/kg, ip) and D-GalN (400 mg/kg). 5 h later, mice were anesthetized with ether and retro-orbitally bled. ALT, AST, TNFα, and IL-6 were measured with ELISA. Also, RNA was extracted from liver tissue and relative mRNA of Tnf, Il6, and Il12b were measured by RT-PCR and Real-time PCR. Mice were monitored for 24 h to assess survival.
Tissue samples collection and evaluation
Pathological analysis of lung, liver and kidney from Nur77+/+ and Nur77−/− mice was conducted and tissue samples were fixed in 10 % buffered formalin and then embedded in paraffin. Tissue was sectioned and stained with hematoxylin and eosin (H&E) according to standard histological procedures.
Real-time PCR assays
Real-time PCR assays were performed as published [7, 18]. The abundance of each mRNA was normalized relative to PCR with the housekeeping gene β-actin. The primers for PCR reactions are listed in Table 1.
ELISA
ALT, AST, TNFα, and IL-6 in serum were measured with commercially available kits according to the manufacturer’s instructions.
Western blot
Western blot analyses were performed as described in the literature [7].
Immunoprecipitation and ubiquitination Assay
Cells were lysed in lysis buffer (2 mmol/L Tris–HCl (pH 7.4), 10 mmol/L EDTA, 100 mmol/L NaCl and 1 % IGEPAL). Cell lysates were incubated with indicated antibodies in protein A/G beads (Santa Cruz Biotechnology) for 3 h. Then, the protein-antibody complexes on the beads were analyzed with Western blot. To measure TRAF6 ubiquitination, 10 mM N-ethylmaleimide (Sigma) was included in the lysis buffer.
Statistical analysis
Data are expressed as means ± SD, and Student’s t test (unpaired, two-tailed) was used to compare two groups of independent samples (p < 0.05 were considered statistically significant).
Results and discussion
Research suggests that orphan nuclear receptor Nur77 is implicated in inflammation and immunity. Mice lacking all Nr4a receptors including Nur77 did not generate Treg cells and resulted in systemic autoimmune disease [19]. Nur77 deficiency in mice lead to acceleration of atherosclerosis [10] and inflammatory bowel disease [6]. We recently reported that loss of Nur77 in older mice contributes to systemic inflammation [20]. Sepsis, the major complication of severe infection, usually causes multisystem organ failure and even death of many patients in hospital [21]. Therefore, it is of great importance to find the new potential targets for sepsis treatment. Here we investigate in vivo functions of Nur77 in sepsis and sepsis-associated liver injury. We first challenged Nur77−/− mice with LPS to ascertain the role of Nur77 in LPS-induced sepsis in vivo. After treatment with LPS, lungs of Nur77−/− mice had severe inflammatory hyperemia, as evidenced by increased mononuclear cells and erythrocyte infiltration (Fig. 1a). RT-PCR and Real-time PCR assays, in liver tissues from Nur77−/− mice, showed substantial induction of Tnf and Il6 expression (Fig. 1b). Consistent with these results, levels of TNFα and IL-6 in serum were significantly higher in LPS-treated Nur77−/− mice than in LPS-treated Nur77+/+ mice (Fig. 1c), suggesting that the host response in Nur77−/− mice is altered. After lethal challenge with LPS, Nur77−/− mouse survival was reduced (Fig. 1d). These results are consistent with a recent report that mice lacking Nur77 had exacerbated inflammatory and immune responses, and survival was decreased after lethal endotoxemic challenge [22]. Thus, Nur77 is important for modulation of inflammatory responses during sepsis.
Mice deficient in Nur77 are more susceptible to LPS-induced sepsis. a Histological analysis of lungs from Nur77+/+ and Nur77−/− mice 6 h after challenge with PBS or LPS (20 mg/kg). Representative images are shown. Scale bars, 100 μM. Original magnification, 100x. b RT-PCR (left) and Real-time PCR (right) quantification of expression of hepatic Tnf and Il6 mRNA from Nur77+/+ and Nur77−/− mice 2 h after challenge with PBS or LPS (20 mg/kg). Error bars represent means ± SD from 3 biological replicates. *p < 0.05 and **p < 0.01. c ELISA assay of TNFα and IL-6 expression in serum of LPS-treated Nur77+/+ mice (n = 5) and LPS-treated Nur77−/− mice (n = 5). Error bars represent means ± SD. **p < 0.01. d Survival of Nur77+/+ mice (n = 7) and Nur77−/− mice (n = 8) treated with LPS and monitored for up to 24 h
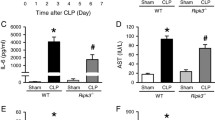
To further ascertain the role of Nur77 in sepsis, we treated mice with LPS/D-GalN known to induce acute liver injury. At 5 h after LPS/D-GalN challenge, Nur77−/− mice had severe hepatocyte destruction compared to wild-type mice (Fig. 2a). LPS/D-GalN injection also promoted hepatocyte cell death in Nur77−/− mice revealed by PARP cleavage (Fig. 2b). ALT and AST, liver function markers, were also significantly greater in serum from LPS/D-GalN-treated Nur77−/− mice (Fig. 2c), indicating increased liver necrosis. We also measured TNFα and IL-6 expression as these are known to be involved in this model of acute liver inflammation [23, 24] in liver tissues from wild-type and Nur77−/− mice. RT-PCR and real-time PCR assays confirmed that expression of these pro-inflammatory cytokines mRNA was greater in LPS/D-GalN-treated Nur77−/− mice than in wild-type mice (Fig. 2d). Similarly, generation of inflammatory cytokines including TNFα and IL-6 were markedly enhanced in serum from Nur77−/− mice (Fig. 2e). Thus, Nur77 is protective against LPS-induced acute liver injury.
Nur77−/− mice have increased susceptibility to LPS/D-GalN-induced acute liver injury. a H&E staining of livers from Nur77+/+ and Nur77−/− mice 5 h after injection with LPS/D-GalN. Representative images are shown. Scale bars, 100 μM. Original magnification, 100x. b Liver extracts assessed by Western blot and indicated antibodies. c ELISA of serum ALT and AST from Nur77+/+ and Nur77−/− mice 5 h after injection with LPS/D-GalN or PBS. Error bars represent means ± SD. *p < 0.05 and **p < 0.01. d Tnf, Il6 and Il12 mRNA expression was measured with RT-PCR (left) and qPCR (right) in livers from Nur77+/+ and Nur77−/− mice 2 h after treatment with LPS/D-GalN. Error bars represent means ± SD from 3 biological replicates. *p < 0.05 and **p < 0.01. e ELISA quantification of serum TNFα, IL-6 from Nur77+/+ and Nur77−/− mice after treatment with LPS/D-GalN for 5 h. Error bars represent means ± SD. *p < 0.05
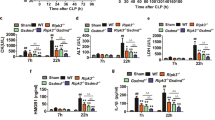
The molecular mechanism by which Nur77 deficiency promotes sepsis remains obscure. Nur77 has been shown to inhibit LPS-induced inflammation by inhibiting p65 binding to DNA, thereby reducing pro-inflammatory cytokine production [25]. We observed enhanced phosphorylation and degradation of IκBα was in liver and spleen tissues from Nur77−/− mice challenged with LPS (Fig. 3a), indicating Nur77 could suppress LPS-induced NF-κB activity in vivo. Also Nur77 contributes to regulation of TRAF6 signalling through its interaction with TRAF6. As shown in Fig. 3b, mice challenged with LPS for 1 h had enhanced Nur77-TRAF6 interaction in the liver and spleen compared to control PBS-treated mice. These results are consistent with our recent observation that disruption of Nur77-TRAF6 interaction in Nur77−/− mice accelerated the development of IBD [6]. Collectively, these data suggest that Nur77 physically interacts with TRAF6 in in vivo mouse model of sepsis, revealing a pathophysiological significance of Nur77-TRAF6 interaction in sepsis. Auto-ubiquitination of TRAF6 is required for NF-κB signal transduction [16]. Here, our results showed that Nur77 deficiency significantly enhanced auto-ubiquitination of TRAF6 in liver and spleen tissues prepared from Nur77−/− but not wild-type mice (Fig. 3c). At the same time, overexpression of Nur77 significantly impaired LPS-induced TRAF6 auto-ubiquitination (Fig. 3d). Also we investigated whether Nur77 can affect auto-ubiquitination of TRAF3, another member of the TRAF family but we observed no significant change. Additional file 1: Figure S1 indicates that overexpression of Nur77 did not affect TRAF3 auto-ubiquitination induced by LPS, suggesting that Nur77 is important in regulating LPS-induced inflammation by targeting TRAF6.
Nur77 inhibits inflammatory response in in vivo mouse model of sepsis by interacting with TRAF6 and regulating TRAF6 auto-ubiquitination (a) Immunoblot analysis of indicated signalling proteins in liver (left) and spleen (right) from Nur77+/+ and Nur77−/− mice 2 h after LPS (20 mg/kg) challenge. b Immunoprecipitation and immunoblot of liver and spleen proteins from C57BL/6 mice 1 h after LPS (20 mg/kg) treatment. c Immunoprecipitation of endogenous TRAF6 from liver (left) and spleen (right) lysates of LPS-treated Nur77+/+ and Nur77−/− mice, and immunoblot of TRAF6 auto-ubiquitination with anti-ubiquitin antibody. d Immunoprecipitation of endogenous TRAF6 from lysates of LPS-treated (50 ng/ml) RAW264.7 cells expressing vector or myc-tagged Nur77 plasmid and immunoblotted for TRAF6 auto-ubiquitination with an anti-ubiquitin antibody
In summary, our in vivo study confirmed a critical protective role for the orphan nuclear receptor Nur77 in sepsis and identify a key mechanism for Nur77 in the regulation of TRAF6 signalling through its interaction with TRAF6 in in vivo mouse model of sepsis.
Abbreviations
- ELISA:
-
Enzyme Linked Immunosorbent Assay
- TNFα:
-
Tumor Necrosis Factor alpha
- IL-6:
-
Interleukin-6
- IL-12:
-
Interleukin-12
References
Wilson TE, Fahrner TJ, Johnston M, Milbrandt J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science. 1991;252:1296–300.
Philips A, Lesage S, Gingras R, Maira MH, Gauthier Y, Hugo P, et al. Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Mol Cell Biol. 1997;17:5946–51.
Zhang XK. Targeting Nur77 translocation. Expert Opin Ther Targets. 2007;11:69–79.
Arkenbout EK, de Waard V, van Bragt M, van Achterberg TA, Grimbergen JM, Pichon B, et al. Protective function of transcription factor TR3 orphan receptor in atherogenesis: decreased lesion formation in carotid artery ligation model in TR3 transgenic mice. Circulation. 2002;106:1530–5.
De Silva S, Han S, Zhang X, Huston DP, Winoto A, Zheng B. Reduction of the incidence and severity of collagen-induced arthritis by constitutive Nur77 expression in the T cell lineage. Arthritis Rheum. 2005;52:333–8.
Wu H, Li XM, Wang JR, Gan WJ, Jiang FQ, Liu Y, et al. NUR77 exerts a protective effect against inflammatory bowel disease by negatively regulating the TRAF6/TLR-IL-1R signalling axis. J Pathol. 2016;238(3):457–69.
Wang JR, Gan WJ, Li XM, Zhao YY, Li Y, Lu XX, et al. Orphan nuclear receptor Nur77 promotes colorectal cancer invasion and metastasis by regulating MMP-9 and E-cadherin. Carcinogenesis. 2014;35(11):2474–84.
Bonta PI, van Tiel CM, Vos M, Pols TW, van Thienen JV, Ferreira V, et al. Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler Thromb Vasc Biol. 2006;26:2288–94.
Pei L, Castrillo A, Tontonoz P. Regulation of macrophage inflammatory gene expression by the orphan nuclear receptor Nur77. Mol Endocrinol. 2006;20:786–94.
Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110:416–27.
Kondo T, Kawai T, Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012;33:449–58.
Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–24.
Deng L, Wang C, Spencer E, Yang L, Braun A, You J, et al. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–61.
Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–5.
Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–33.
Wang Y, Tang Y, Teng L, Wu Y, Zhao X, Pei G. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2006;7:139–47.
Wu Y, Liu J, Zhang Z, Huang H, Shen J, Zhang S, et al. HSP27 regulates IL-1 stimulated IKK activation through interacting with TRAF6 and affecting its ubiquitination. Cell Signal. 2009;21:143–50.
Xing C, Lu XX, Guo PD, Shen T, Zhang S, He XS, et al. Ubiquitin-Specific Protease 4-Mediated Deubiquitination and Stabilization of PRL-3 Is Required for Potentiating Colorectal Oncogenesis. Cancer Res. 2016;76(1):83–95.
Sekiya T, Kashiwagi I, Yoshida R, Fukaya T, Morita R, Kimura A, et al. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat Immunol. 2013;14:230–7.
Li XM, Lu XX, Xu Q, Wang JR, Zhang S, Guo PD, et al. Nur77 deficiency leads to systemic inflammation in elderly mice. J Inflamm (Lond). 2015;12:40.
Lever A, Mackenzie I. Sepsis: definition, epidemiology, and diagnosis. BMJ. 2007;335:879–83.
Uhrin P, Perkmann T, Binder B, Schabbauer G. ISG12 is a critical modulator of innate immune responses in murine models of sepsis. Immunobiology. 2013;218:1207–16.
Leist M, Gantner F, Jilg S, Wendel A. Activation of the 55 kDa TNF receptor is necessary and sufficient for TNF-induced liver failure, hepatocyte apoptosis, and nitrite release. J Immunol. 1995;154:1307–16.
Rikans LE, DeCicco LA, Hornbrook KR, Yamano T. Effect of age and carbon tetrachloride on cytokine concentrations in rat liver. Mech Ageing Dev. 1999;108:173–82.
Li L, Liu Y, Chen HZ, Li FW, Wu JF, Zhang HK, et al. Impeding the interaction between Nur77 and p38 reduces LPS-induced inflammation. Nat Chem Biol. 2015;11:339–46.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81372574, 31300630, 81525020, 31570753 and 31540036), Natural Science Foundation of Jiangsu Province (BK20130337), China Postdoctoral Science Foundation funded project (2013 M541727 and 2014 T70546), and Postdoctoral Science Foundation of Jiangsu Province (1302154C). This work was also supported by a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors’ declare that they have no competing interests.
Authors’ contributions
HW and JML conceived of and supervised the study. HW, XML and JML designed the experiments and analyzed data. XML, SZ, XSH, PDG, XXL and JRW performed the experiments. HW and JML wrote the manuscript. All authors read and approved the final manuscript.
Xiu-Ming Li, Shen Zhang and Xiao-Shun He are co-first authors.
Additional file
Additional file 1: Figure S1.
Nur77 does not affect auto-ubiquitination of TRAF3 induced by LPS. Immunoprecipitation of endogenous TRAF3 from lysates of LPS-treated (50 ng/ml) RAW264.7 cells expressing vector or myc-tagged Nur77 plasmid, immunoblotted for TRAF3 auto-ubiquitination with an anti-ubiquitin antibody. (PDF 62 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, XM., Zhang, S., He, XS. et al. Nur77-mediated TRAF6 signalling protects against LPS-induced sepsis in mice. J Inflamm 13, 4 (2016). https://doi.org/10.1186/s12950-016-0112-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12950-016-0112-9