Abstract
The gut microbiota can significantly affect the function of the intestinal barrier. Some intestinal probiotics (such as Lactobacillus, Bifidobacteria, a few Escherichia coli strains, and a new generation of probiotics including Bacteroides thetaiotaomicron and Akkermansia muciniphila) can maintain intestinal epithelial homeostasis and promote health. This review first summarizes probiotics’ regulation of the intestinal epithelium via their surface compounds. Surface layer proteins, flagella, pili and capsular polysaccharides constitute microbial-associated molecular patterns and specifically bind to pattern recognition receptors, which can regulate signaling pathways to produce cytokines or inhibit apoptosis, thereby attenuating inflammation and enhancing the function of the gut epithelium. The review also explains the effects of metabolites (such as secreted proteins, organic acids, indole, extracellular vesicles and bacteriocins) of probiotics on host receptors and the mechanisms by which these metabolites regulate gut epithelial barrier function. Previous reviews summarized the role of the surface macromolecules or metabolites of gut microbes (including both probiotics and pathogens) in human health. However, these reviews were mostly focused on the interactions between these substances and the intestinal mucosal immune system. In the current review, we only focused on probiotics and discussed the molecular interaction between these bacteria and the gut epithelial barrier.

Similar content being viewed by others
Background
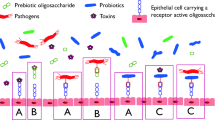
The gut is a diversiform microenvironment in which hundreds of types of bacteria grow [1]. Intestinal epithelial cells (IECs) are generally considered to be immune sentinels and to play a crucial role in maintaining the integrity of the host’s intestinal mucosa [2]. Structurally, the monolayer of IECs separates the mucus produced by the goblet cells and the microbiota from the underlying immune cells to form a gut epithelial barrier (Fig. 1) [3]. The intestinal epithelial barrier is thus the main defense mechanism against infection and inflammation, and the disruption of its integrity is one of the primary causes of several intestinal disorders [4], including inflammatory bowel disease, necrotizing enterocolitis, diabetes, obesity, and irritable bowel syndrome [5]. Although gut diseases have a certain relationship with factors such as diet, genetics, and the environment, it is generally believed that dysbacteriosis is the most important factor that affects the intestinal barrier [6].
Structure, function, and probiotics of intestinal epithelial barrier. The mucus secreted by goblet cells continuously replenishes the mucosal layer that covers the intestinal epithelium, which acts as the first physical barrier against pathogenic bacteria. The symbiotic bacteria in the outer mucus layer can ferment dietary fiber into SCFAs, providing important energy sources for colonic intestinal cells and goblet cells. Paneth cells secrete a variety of antibacterial substances, such as antimicrobial peptides and Reg3γ. These antibacterial substances and secretory IgA are secreted into mucus to protect against commensal pathogens. The microorganism-associated molecular patterns (MAMPs) of probiotics can be recognized by PRRs such as TLRs, which induces the response of dendritic cells (DCs) to provide the protection on gut epithelial barrier. PRRs pattern recognition receptors, SCFAs short-chain fatty acids
Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the consumer” [7]. Commonly recognized intestinal probiotics include Lactobacillus, Bifidobacterium, Streptococcus, and a few Escherichia coli strains [1]. Recent studies have also indicated that some intestinal symbiotic bacteria such as Akkermansia muciniphila and Bacteroides thetaiotaomicron demonstrate the potency to comprise a new generation of probiotics [8, 9]. These bacteria have long been proven to regulate intestinal epithelial function by facilitating the formation of mucous layers, secreting antibacterial factors, boosting the secretion of secretory immunoglobulin A (SlgA) and competitive adhesion to intestinal epithelial cells [10, 11], and increasing tight junction formation [12]. Although these protective effects have been well documented, the underlying molecular mechanism of probiotics on the gut barrier has not been thoroughly reviewed.
The surface components of probiotics, such as flagella, pili, surface layer proteins (SLPs), capsular polysaccharide (CPS), lipoteichoic acid, and lipopolysaccharide, constitute microbial-associated molecular patterns (MAMPs) [13]. They can specifically bind to pattern recognition receptors (PRRs) such as NOD-like receptors (NLRs) and toll-like receptors (TLRs) (Table 1) [14, 15], and regulate nuclear factor kappa B (NF-κB), mitogen-activated protein kinases (MAPK), peroxisome proliferator-activated receptor gamma, and other signaling pathways in IEC [16]. MAMPs also regulate a cellular protease-dependent signaling cascade to produce a variety of cytokines and chemokines that alleviate inflammation and enhance intestinal epithelial function [10, 17]. In addition, some metabolites produced by probiotics, such as secreted proteins (extracellular proteins), organic acids, indole, bacteriocins, H2O2, and NO, protect the gut’s epithelial barrier by boosting mucus secretion by goblet cells, increasing the production of antimicrobial peptides, or enhancing the expression of tight junctions (Fig. 1) [18].
Based on the above mentioned analyses on the potential role of the surface compounds and metabolites of probiotics in gut barrier function, [10,11,12,13, 18] this review provides updated and comprehensive information on the molecular interaction between intestinal probiotics and the gut barrier and summarizes the effects of the surface macromolecules and metabolites of probiotics on intestinal receptors and pathways.
Regulation of intestinal barrier function by surface molecules of probiotics
A number of previous studies have shown that the surface molecules of probiotics including SLPs, flagella, fimbriae and CPS can be recognized by PRRs and play a role in maintaining intestinal homeostasis and promoting gut health (Fig. 2) [13, 14, 16].
Effects of surface molecular of probiotics on intestinal epithelial barrier. Flagellin, pili, and CPS can be bind to TIR domain in TLRs, thus interacting with adaptor molecules such as MyD88 to activate AP-1 and NF-κB signaling pathways in IEC. Flagellin of EcN can finally induce the expression of HBD-2 in the gut, which is beneficial for the prevention of pathogens. F1C pili of EcN can finally up-regulate the expression of tight junction to enhance gut barrier function. CPS of EcN can finally induce the secretion of cytokines such as IL-10 and IL-12 for the alleviation of intestinal inflammation. SlpA of Lactobacillus acidophilus can bind to DC-SIGN and increase ERK phosphorylation, which mediates interaction with NF-κB and then reduce the expression level of cell apoptosis. SLPs surface layer proteins, CPS capsular polysaccharide, TLRs toll-like receptors, DC-SIGN dendritic cell specific intercellular adhesion molecule grabbing nonintegrin, NF-κB nuclear factor kappa B, AP-1 activating protein-1, IECs intestinal epithelial cells, ERK extracellular signal-regulated kinase, MAPK mitogen-activated protein kinase, HBD-2 beta-defensin 2
Surface layer proteins
Bacterial surface layers are supramolecular cell envelope structures that are abundant in Archaea and in Gram-negative and Gram-positive bacteria [19, 20]. Chemical analyses of isolated S-layers showed that they are mostly composed of a single species of protein or multiple species of glycoproteins, with apparent relative molecular weights of 40,000 to 200,000 [21, 22]. These proteins were named as S-layer proteins (SLPs) [21, 22]. SLPs form a regular lattice monolayer via self-assembly and attach to the extracellular membrane by noncovalent interactions [21, 23]. As the outermost structure of the cell, the surface layer lattice is generally considered to be the first bacterial components that have a direct interaction with the host’s epithelium.
In previous studies, L. helveticus R0052 inhibited the adhesion of E. coli O157:H7 to Caco-2 cells [24], and its surface protein extract was able to co-aggregate with Salmonella typhimurium FP1 [25]. The function of SLPs in bacterial adhesion and gut barrier protection can be attributed to SLPs’ competition with pathogens such as enterohemorrhagic E. coli (EHEC), enteroinvasive E. coli (EIEC) and enteropathogenic E. coli (EPEC) for adhesion sites on the intestinal cell surface. It can also be attributed to their surface hydrophobicity [26], surface charge distribution [27], and co-aggregation of pathogenic bacteria [19].
A recent study indicated that purified SLPs from L. plantarum exert a protective effect on Caco-2 cells infected with EPEC by increasing their transepithelial resistance (TER) and down-regulating their permeability [28]. The SLPs of L. acidophilus have also been reported to protect the intestinal epithelium and inhibit its invasion by Salmonella enterica serovar Typhimurium by recovering TER [29]. SLPs can protect the intestinal barrier by affecting F-actin distribution and modulating the tight junction proteins at the mRNA and protein levels [30]. They can also increase extracellular signal-regulated kinase (ERK) phosphorylation, reducing the level of cell apoptosis [28].
Micro integral membrane proteins (MIMPs) were identified as the smallest domain from the SLPs of L. plantarum [31]. Previous studies have shown that MIMPs of L. plantarum CGMCC 1258 can restore tight junctional injury by increasing the expression of tight junction proteins including JAM-1, occludin, and claudin-1, which can allow the transportation of ions and small molecules of soluble substances through gut barrier, but prevent the passage of toxic large molecules and microorganisms [32].
Flagellin
Flagellin is a structural component of bacterial flagella produced by pathogenic, symbiotic bacteria and neutral bacteria [33]. The interaction between flagellin and intestinal epithelium has mostly been studied on E. coli Nissle 1917 (EcN) [34]. Flagellin can induce inflammation in intestinal epithelial cells, whereas this proinflammatory effect is dismissed without contact with the basolateral membrane of the gut epithelia. This explains why flagellin-producing symbiotic microbes have not been found to induce inflammation in the gut lumen [35]. It has been reported that flagellin serves to activate phosphatidylinositol-3-kinase (PI3K)/AKT signaling pathway in the gut epithelium via a TLR5-dependent mechanism [36, 37]. The rapid activation of the PI3K pathway by TLR5 can limit the MAPK signaling pathway, thereby limiting the expression of proinflammatory genes and inhibiting inflammation [37]. It has also been reported that flagellin produced by the EcN can induce the secretion of beta-defensin 2 (HBD-2) [38], an antimicrobial peptide synthesized by intestinal epithelial cells. A follow-up study showed that the flagella-induced induction of HBD-2 is related to the NF-κB and activating protein-1 (AP-1) signaling pathways and thus offers antagonism against pathogens [34, 39]. It has been reported that the flagellum of the EcN, a main adhesin of intestinal mucous, can bind to receptors such as the mucus component gluconate and mediate its adhesion to mucin 2 [40]. These action modes can exclude pathogens and protect the intestinal epithelial barrier.
Pili
Pili is a filamentous accessory organ on the surface of bacteria, which plays an important role in the adhesion between bacteria and host’s intestinal epithelium [41]. Pili is divided into 6 types (type I–type VI), based on their morphology, number, distribution on the surface of bacteria, adhesion characteristics, antigenicity and genetic locus [41]. Studies have revealed that EcN produces three main kinds of adhesins: F17-like pili, type 1 pili, and F1C pili [42]. Both F17-like and type 1 pili contribute to intestinal colonization and show significant binding to the epithelium in mice [42]. F1C pili can attach to mannosylated glycoproteins in the intestine and motivate TLR4 in a MyD88-dependent manner, thus improving the colonization and biofilm formation of EcN in the gut [42].
In vitro and in vivo experiments have demonstrated that the tight adhesion (Tad) pili of B. breve UCC2003 is a subclass of the type IVb pili. Tad has been reported to promote the proliferation of intestinal epithelial cells in mice [43]. The probiotic effect of Bifidobacterium Tad pili on the intestinal epithelial barrier can stimulate neonatal mucosal growth and intestinal maturation by producing a specific extracellular protein structural scaffold [44]. Subsequent reports have revealed that this beneficial proliferation response depends largely on the pili subunit TadE [44]. It has also been shown that SpaC fimbriae of probiotics are essential for adhesion to Caco-2 intestinal epithelium lines [45, 46]. The SpaC pilin of L. rhamnosus GG (LGG) has been confirmed to induce the generation of reactive oxygen species (ROS) in epithelium and play a role in stimulating ERK phosphorylation and protecting the gut’s epithelial barrier [47].
Capsular polysaccharide
The CPS of bacteria is homopolymers or heteropolymers formed by repeated monosaccharides linked by glycosidic bonds [19]. CPS molecules in probiotics have a positive effect on adaptation to the intestinal microenvironment. B. thetaiotaomicron can express and dynamically transform various types of CPS in vivo, the most prevalent being CPS5, which can enhance the competition and colonization of bacteria in the gut of mice [48]. CPS5 also enhances the tolerance of B. thetaiotaomicron to antibiotic stress [48]. Furthermore, some studies revealed that the K5 capsule of EcN stimulates TLR5 in gut epithelial cells and induces chemokine expression via the mitogen-activated protein kinase pathway [49, 50].
To summarize, the surface substances of probiotics share a common regulatory mechanism as they can bind to PRRs including TLRs, NLRs, DC-SIGN and CLRs. Upon exposure to these surface substances, PRRs respond by activating associated adaptor proteins that are linked to NF-κB and MAPK signaling cascades, which further affects the expression of genes encoding cytokines, chemokines and antimicrobial peptides.
Regulation of intestinal barrier function by main metabolites of probiotics
Some metabolites produced by probiotics, such as secreted proteins (extracellular proteins), indole, extracellular vesicles, short-chain fatty acids, and bacteriocins also protect the intestinal epithelial barrier by interacting with some receptors or directly promoting mucus secretion by goblet cells, increasing the secretion of antimicrobial peptides, or enhancing the expression of tight junctions [18].
Secreted protein of probiotics
A number of previous studies indicated that secreted proteins (extracellular proteins) are proteins secreted and released into the environment by probiotic [51,52,53]. The secreted proteins of probiotics have also been reported to participate in the interaction between symbiotic bacteria and the host. The extracellular proteins secreted by L. plantarum BMCM12 effectively attenuate the adherence of pathogens and protect the intestinal barrier [51]. Two proteins produced by LGG, p40 and p75, have been shown to promote IEC homeostasis. The mechanism is as follows. First, the soluble proteins P75 and p40 transactivate the epidermal growth factor receptor (EGFR) [52] and then up-regulate the expression of a proliferation-inducing ligand (APRIL) in the epithelium (Fig. 3) [53]. This in turn promotes the production of immunoglobulin A and attenuates cytokine-induced apoptosis in mouse small intestine epithelial cells [53]. Second, these two proteins stimulate the intestinal epithelial cells to produce protective heat stress proteins Hsp72 and Hsp25, which protect tight junction proteins and activate the Akt pathway in a phosphatidylinositol 3-kinase (PIK3)-dependent manner to enhance the proliferation and survival of gut epithelial cells (Fig. 2) [54]. Alternatively, other studies have demonstrated that neonatal supplementation of P40 and p75 can promotes intestinal development and prevents colitis in adulthood [55, 56]. Moreover, these two proteins also prevent H2O2-induced tight junctional disruption by protein kinase C (PKC)-dependent mechanisms [57].
Effects of metabolites of probiotics on intestinal epithelial barrier. Indole 3-propionic acid can bind to PXR and up-regulate the expression of tight junction protein. The indole-3-lactic acid activates AhRs of the gut epithelium and promotes the expression of IL-22. The soluble proteins P40 and p75 isolated from LGG can activate EGFR and then up-regulate the expression of an APRIL in the epithelium, thus stimulating the secretion of lgA by B cells. Besides, P40 and p75 can activate EGFR–PIK3–Akt signaling pathway to maintain gut homeostasis. Moreover, these two proteins also prevent tight junctional disruption by protein kinase C (PKC)-dependent mechanisms. Butyrate is able to bind to the GPCR including GPR41, GPR109A, and GPR43 and induce the production of IL-18 in the colonic epithelium. Furthermore, butyrate also motivates the O2 consumption of the gut epithelium to maintain HIF stability and increase the expression of barrier-protective HIF target genes. In addition, bacteriocins produced by probiotics act as colonizing peptides to encourage producers to gain a competitive advantage over other strains and to occupy established niches in the intestines. Alternatively, bacteriocins can act as a killing peptide, directly inhibiting the adhesion of pathogens to the mucus layer and protecting the first barrier of the intestinal tract. HIF hypoxia-inducible factor, GPR109A G-protein-coupled receptors 109A, AhRs aryl hydrogen receptors, P75 and P40 cell wall-associated hydrolase, EGFR epidermal growth factor receptor, PI3K phosphatidylinositol-3-kinase, PKC protein kinase C, PXR pregnane X receptor, APRIL a proliferation-inducing ligand, PKC protein kinase C
Similarly, a novel LGG-soluble protein HM0539, has been reported to protect intestinal integrity by mediating tight junction expression and mucus secretion [58]. Furthermore, Ewaschuk et al. used a mouse model with and without interleukin (IL)-10 and found that an extracellular protein secreted by B. infantis positively regulated occludin and ZO-1 proteins and increased TER, thus reducing colonic permeability and strengthening the mucosal barrier [59].
Indole
Indole is usually produced by bacteria that contain tryptophanase and has been reported to be a specific intestinal symbiotic bacteria signal [60, 61]. Studies have indicated that indole produced by symbiotic E. coli can inhibit the chemotaxis of pathogenic E. coli [62]. E. coli-secreted indole can also inhibit the attachment of pathogens to the epithelium by increasing the expression of genes involved in intestinal epithelial function, such as actin cytoskeleton, adhesion junctions, and tight junctions [63]. Furthermore, this bacterial signal increased TER in polarized HCT-8 gut epithelium and attenuated tumor necrosis factor α-mediated NF-κB activation and IL-8 secretion, thus facilitating epithelial function [63].
The pregnane X receptor (PXR) is a physiologic regulator associated with gut permeability, which is considered to regulate the intestinal barrier mediated by TLR4 [64,65,66]. Indole 3-propionic acid (IPA) has been reported as a ligand for epithelial PXR [61, 67], and the administration of IPA can up-regulate tight junction protein-coding mRNAs and augment the expression of claudins and occludins [65]. It has been reported that the indole-3-lactic acid produced by B. infantis activates the aryl hydrogen receptors (AhRs) of the gut epithelium by increasing their nuclear localization and up-regulating the protein expression of CYP1A1 [68]. The activation of AhRs then leads to lL-22 transcription, which can further increase the expression of antimicrobial peptides and improve colonization resistance against Candida albicans in the gastrointestinal tract [68].
Extracellular vesicles
Extracellular vesicles (EVs), nanoscale membrane vesicles, are lipid bilayer structures secreted by the intestinal microbiota that are composed mainly of nucleic acids, proteins, lipids, and lipopolysaccharides [69, 70]. EVs are involved in bacteria-host communication and in the maintenance of gut homeostasis. It has been reported that oral application of A. muciniphila derived EVs can alleviate dextran sulfate sodium-induced colitis by recovering inflammatory cell infiltration of the colon wall and alterations in colon length [71]. These phenomena can be explained by the fact that A. muciniphila derived EVs up-regulate the expression of claudin-3 and reduce intestinal permeability in diabetic mice in an AMP-activated protein kinase (AMPK)-dependent manner [72,73,74].
The EVs of most bacteria are obtained by blistering the outer membrane and ultimately pinching off the bacterial cytoderm, so they are referred to as outer membrane vesicles (OMVs). Studies have shown that OMVs secreted by E. coli ECOR63 and EcN can upregulate tight junction proteins such as claudin-14 and ZO-1 [75, 76]. Probiotic EcN derived OMVs can also induce IL-22 expression in colonic explants, thereby preventing allergens and pathogenic microorganisms from entering the systemic circulation [75].
Short-chain fatty acids
Short-chain fatty acids, which comprise mainly butyrate, propionate, and acetate, are metabolites secreted by intestinal microbiota from undigested dietary carbohydrates and proteins [77]. As butyrate is the preferential source of energy for colonic epithelial cell among all short-chain fatty acids, the relationship between butyrate and the intestinal epithelial barrier is the most-studied [78].
Studies have revealed the protective effect of a low concentration of butyrate (≤ 2 mM) on the single-layer barrier of Caco-2 cells, such as the increase in TER and the decrease in inulin permeability [79, 80]. Moreover, microbial-derived butyrate boosts the expression of tight junction proteins and represses paracellular permeability in vivo [81], and it stimulates goblet cells to secrete mucin, especially MUC2, which prevents pathogenic bacteria from destroying enterocytes [82]. A mucin-related peptide that can repair the intestinal mucosa, trefoil factor, can also be upregulated by butyrate [77]. Butyrate contributes to activate hypoxia-inducible factor (HIF) in the hypoxic region of the colon, which further promotes intestinal epithelial barrier function, antimicrobial defense, and mucus production [83, 84].
Butyrate is a histone deacetylase inhibitor and has been reported to bind to specific G-protein-coupled receptors, including GPR109A, GPR43, and GPR41 [85, 86]. Of these, GPR109A is crucial for the production of IL-18 in the colonic epithelium and has been confirmed to have an important effect on the maintenance of intestinal homeostasis (Fig. 3) [81, 87]. One of the mechanisms by which butyrate improves gut epithelial barrier function is the activation of AMP-activated protein kinase [87, 88]. Second, low concentrations of butyrate can augment the MUC2 mRNA level by promoting AP-1 binding to the MUC2 promoter [82]. At the same time, butyrate can boost the acetylation of histones H4 and H3 and the methylation of H3 on the MUC2 promoter, thereby safeguarding the mucosal barrier [82]. Butyrate also inhibits permeability-promoted claudin-2 tight junction protein expression via an IL-10RA-dependent mechanism [89]. Furthermore, the production of antimicrobial cathelicidin, such as LL-37 in the body has also been specifically linked to butyrate [90]. In addition, butyrate can motivate the O2 consumption of the gut epithelium to the extent of HIF stability and increase the expression of barrier-protective HIF target genes, connecting microbes and epithelial barriers (Fig. 3) [91, 92].
Bacteriocins
Bacteriocins are a class of ribosomally synthesized antimicrobial peptides [93,94,95] and can be divided into two specific classes: lanthionine-containing bacteriocins/lbacteria (class I) and non-lanthionine-containing bacteriocins (class II). [96]. The class I bacteriocins comprise single peptide chain and polypeptide chain lantibiotics. These bacteriocins, including lacticin 481, lacticin 3147, and nisin, are ribosomally-synthesised antimicrobial peptides produced by Gram-positive bacteria. [97, 98]. The class II bacteriocins are mainly composed of subclass I, subclass II, subclass III and subclass IV. The common bacteriocins in class II are pediocin pa-1, lactacin F, lactococcin A and reuterin 6. We have added an introduction to the classification of bacteriocins [99].
Bacteriocins have been reported to act as colonizing peptides of certain intestinal micro-organisms, promoting these bacteria to acquire a competitive advantage over other strains and occupy established niches in the intestines [100]. Studies have shown that EcN can secrete microcin H47 and microcin M, two antimicrobial peptides with low molecular weight that can be discerned by the catecholate siderophore receptors and thus enhance the competitiveness of EcN with other microorganisms [101]. Bacteriocin produced by the strain Enterococcus faecium KH24 conspicuously affects the microbiome in the feces of mice [102]. In addition to reducing the number of E. coli, this bacteriocin can significantly increase the abundance of Lactobacillus [102].
Alternatively, bacteriocins function as killing peptides since they can interfere with the growth of pathogens (especially Gram-negative bacteria) by penetrating the inner membrane or disrupting cell wall synthesis. [103]. L. reuteri can secrete a secondary metabolite with broad-spectrum antibacterial activity, called reuterin, that directly inhibits pathogens [104]. Moreover, nisin, which is mainly produced by Streptococcus lactis and Lactococcus lactis, can restrain the growth and reproduction of most Gram-positive bacteria and their spores, especially against S. aureus and Streptococcus hemolyticus [105]. Furthermore, the class II bacteriocin Abp118 secreted by L. salivarius UCC118 can prominently protect mice from infection by Listeria monocytogenes [106]. In addition, EntV produced by E. faecalis bacteria represses hyphae and biofilm formation in Candida albicans and reduce the virulence of this fungus [107].
Conclusions
Probiotics and gut commensals can modulate the host’s gut epithelial barrier function via their surface molecules and metabolites. Through organoid models, sterile animal models, and in vitro tissue, we may better characterize the impact of the intestinal microflora on the host epithelium. Surface components and metabolites of probiotics can be further used in clinical studies and dietary interventions for the treatment of diseases associated with specific intestinal barriers [108,109,110,111,112].
Availability of data and materials
Not applicable.
Abbreviations
- MAMPs:
-
Microbial-associated molecular patterns
- PRRs:
-
Pattern recognition receptors
- NLRs:
-
NOD-like receptors
- TLRs:
-
Toll-like receptors
- NF-κB:
-
Nuclear factor kappa B
- MAPK:
-
Mitogen-activated protein kinases
- SlgA:
-
Secretory immunoglobulin A
- SLPs:
-
Surface layer proteins
- TER:
-
Transepithelial resistance
- ERK:
-
Extracellular signal-regulated kinase
- PI3K:
-
Phosphatidylinositol-3-kinase
- HBD-2:
-
Beta-defensin 2
- EcN:
-
Escherichia coli Nissle 1917
- Tad:
-
Tight adhesion
- CPS:
-
Capsular polysaccharide
- ROS:
-
Reactive oxygen species
- EGFR:
-
Epidermal growth factor receptor
- APRIL:
-
A proliferation-inducing ligand
- PXR:
-
The pregnane X receptor
- IPA:
-
Indole 3-propionic acid
- AhRs:
-
Aryl hydrogen receptors
- EVs:
-
Extracellular vesicles
- OMVs:
-
Outer membrane vesicles
- HIF:
-
Hypoxia-inducible factor
- PKC:
-
Protein kinase C
- Dgk:
-
Diacylglycerol kinase
References
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human Intestinal microbial flora. Science. 2005;308:1635–8.
Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–52.
Ohland CL, MacNaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Liver Physiol. 2010;298:G807–19.
Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol. 2010;7:503–14.
König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, Whyte J, Troost F, Brummer RJ. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. 2016;7:e196.
Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 2017;15:630–8.
Pineiro M, Stanton C. Probiotic bacteria: legislative framework-requirements to evidence basis. J Nutr. 2007;137:850S–3S.
Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. A genomic view of the human-bacteroides thetaiotaomicron symbiosis. Science. 2003;2999:2074–6.
Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066–71.
Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–20.
Patel R, DuPont HL. New approaches for bacteriotherapy: prebiotics, new-generation probiotics, and synbiotics. Clin Infect Dis. 2015;60:S108–21.
Rook G, Bäckhed F, Levin BR, McFall-Ngai MJ, McLean AR. Evolution, human-microbe interactions, and life history plasticity. Lancet. 2017;390:521–30.
Lebeer S, Bron PA, Marco ML, Van Pijkeren JP, O’Connell Motherway M, Hill C, Pot B, Roos S, Klaenhammer T. Identification of probiotic effector molecules: present state and future perspectives. Curr Opin Biotechnol. 2018;49:217–23.
Wells JM, Rossi O, Meijerink M, van Baarlen P. Epithelial crosstalk at the microbiota–mucosal interface. Proc Natl Acad Sci USA. 2011;108:4607–14.
Kleerebezem M, Hols P, Bernard E, Rolain T, Zhou M, Siezen RJ, et al. The extracellular biology of the Lactobacilli. FEMS Microbiol Rev. 2010;34:199–230.
Siciliano RA, Mazzeo MF. Molecular mechanisms of probiotic action: a proteomic perspective. Curr Opin Microbiol. 2012;15:390–6.
Sharma R, Young C, Neu J. Molecular modulation of intestinal epithelial barrier: contribution of microbiota. J Biomed Biotechnol. 2010;2010:305879.
Kumar M, Nagpal R, Verma V, Kumar A, Kaur N, Hemalatha R, Gautam SK, Singh B. Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr Rev. 2013;71:23–34.
Lebeer S, Vanderleyden J, De Keersmaecker SCJ. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010;8:171–84.
Sleytr UB, Beveridge TJ. Bacterial S-layers. Trends Microbiol. 1999;7:253–60.
Albers S-V, Meyer BH. The archaeal cell envelope. Nat Rev Microbiol. 2011;9:414–26.
Fagan RP, Fairweather NF. Biogenesis and functions of bacterial S-layers. Nat Rev Microbiol. 2014;12:211–22.
Abu-Qarn M, Eichler J, Sharon N. Not just for eukarya anymore: protein glycosylation in bacteria and archaea. Curr Opin Struct Biol. 2008;18:544–50.
Sleytr UB, Schuster B, Egelseer E-M, Pum D. S-layers: principles and applications. FEMS Microbiol Rev. 2014;38:823–64.
Johnson-Henry KC, Hagen KE, Gordonpour M, Tompkins TA, Sherman PM. Surface-layer protein extracts from Lactobacillus helveticus inhibit enterohaemorrhagic Escherichia coli O157:H7 adhesion to epithelial cells. Cell Microbiol. 2007;9:356–67.
Beganović J, Frece J, Kos B, Leboš Pavunc A, Habjanič K, Šušković J. Functionality of the S-layer protein from the probiotic strain Lactobacillus helveticus M92. Anton Leeuw Int J G. 2011;100:43–53.
Vadillo-Rodríguez V, Busscher HJ, Norde W, de Vries J, van der Mei HC. Dynamic cell surface hydrophobicity of Lactobacillus strains with and without surface layer proteins. J Bacteriol. 2004;186:6647–50.
Li P-N, Herrmann J, Tolar BB, Poitevin F, Ramdasi R, Bargar JR, Stahl DA, Jensen GJ, Francis CA, Wakatsuki S, van den Bedem H. Nutrient transport suggests an evolutionary basis for charged archaeal surface layer proteins. ISME J. 2018;12:2389–402.
Konstantinov SR, Smidt H, de Vos WM, Bruijns SC, Singh SK, Valence F, Molle D, Lortal S, Altermann E, Klaenhammer TR, van Kooyk Y. S layer protein a of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc Natl Acad Sci USA. 2008;105:19474–9.
Prado Acosta M, Ruzal SM, Cordo SM. S-layer proteins from Lactobacillus sp. inhibit bacterial infection by blockage of DC-SIGN cell receptor. Int J Biol Macromol. 2016;92:998–1005.
Liu Z, Shen T, Zhang P, Ma Y, Qin H. Lactobacillus plantarum surface layer adhesive protein protects intestinal epithelial cells against tight junction injury induced by enteropathogenic Escherichia coli. Mol Biol Rep. 2011;38:3471–80.
Yin M, Yan X, Weng W, Yang Y, Gao R, Liu M, Pan C, Zhu Q, Li H, Wei Q, Shen T, Ma Y, Qin H. Micro integral membrane protein (MIMP), a newly discovered anti-inflammatory protein of Lactobacillus Plantarum, enhances the gut barrier and modulates microbiota and inflammatory cytokines. Cell Physiol Biochem. 2018;45:474–90.
Qin H, Zhang Z, Hang X, Jiang YL. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol. 2009;9:63.
Schlee M, Wehkamp J, Altenhoefer A, Oelschlaeger TA, Stange EF, Fellermann K. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect Immun. 2007;75:2399–407.
Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–5.
Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–103.
Song WS, Jeon YJ, Namgung B, Hong M, Yoon SI. A conserved TLR5 binding and activation hot spot on flagellin. Sci Rep. 2017;7:40878.
Tahoun A, Jensen K, Corripio-Miyar Y, McAteer S, Smith DGE, McNeilly TN, Gally DL, Glass EJ. Host species adaptation of TLR5 signalling and flagellin recognition. Sci Rep. 2017;7:17677.
Cheng HY, Ning MX, Chen DK, Ma WT. Interactions between the gut microbiota and the host innate immune response against pathogens. Front Immunol. 2019;10:607.
Lewis SB, Prior A, Ellis SJ, Cook V, Chan SSM, Gelson W, Schüller S. Flagellin induces β-defensin 2 in human colonic ex vivo infection with enterohemorrhagic Escherichia coli. Front Cell Infect Microbiol. 2016;6:68.
Troge A, Scheppach W, Schroeder BO, Rund SA, Heuner K, Wehkamp J, Stange EF, Oelschlaeger TA. More than a marine propeller-the flagellum of the probiotic Escherichia coli strain Nissle 1917 is the major adhesin mediating binding to human mucus. Int J Med Microbiol. 2012;302:304–14.
Lasaro MA, Salinger N, Zhang J, Wang Y, Zhong Z, Goulian M, Zhu J. F1C fimbriae play an important role in biofilm formation and intestinal colonization by the Escherichia coli commensal strain Nissle 1917. Appl Environ Microbiol. 2009;75:246–51.
O’Connell Motherway M, Zomer A, Leahy SC, Reunanen J, Bottacini F, Claesson MJ, O’Brien F, Flynn K, Casey PG, Munoz JA, Kearney B, Houston AM, O’Mahony C, Higgins DG, Shanahan F, Palva A, de Vos WM, Fitzgerald GF, Ventura M, O’Toole PW, van Sinderen D. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc Natl Acad Sci USA. 2011;108:11217–22.
O’Connell Motherway M, Houston A, O’Callaghan G, Reunanen J, O’Brien F, O’Driscoll T, Casey PG, de Vos WM, van Sinderen D, Shanahan F. A Bifidobacterial pilus-associated protein promotes colonic epithelial proliferation. Mol Microbiol. 2019;111:287–301.
Segers ME, Lebeer S. Towards a better understanding of Lactobacillus rhamnosus GG-host interactions. Microb Cell Fact. 2014;13:S7.
Lebeer S, Verhoeven TLA, Francius G, Schoofs G, Lambrichts I, Dufrêne Y, Vanderleyden J, De Keersmaecker SC. Identification of a gene cluster for the biosynthesis of a long, galactose-rich exopolysaccharide in Lactobacillus rhamnosus GG and functional analysis of the priming glycosyltransferase. Appl Environ Microbiol. 2009;75:3554–63.
Ardita C, Mercante J, Kwon YM, Jones R, Powel D, Neish A. Pilin SpaC-mediated epithelial adhesion is required for Lactobacillus rhamnosus GG-induced probiotic effects. FASEB J. 2014;28:60–9.
Porter NT, Canales P, Peterson DA, Martens EC. A subset of polysaccharide capsules in the human symbiont Bacteroides thetaiotaomicron promote increased competitive fitness in the mouse gut. Cell Host Microbe. 2017;22:494–506.
Hafez M, Hayes K, Goldrick M, Grencis RK, Roberts IS. The K5 capsule of Escherichia coli strain Nissle 1917 is important in stimulating expression of Toll-like receptor 5, CD14, MyD88, and TRIF together with the induction of interleukin-8 expression via the mitogen-activated protein kinase pathway in epithelial cells. Infect Immun. 2010;78:2153–62.
Nzakizwanayo J, Kumar S, Ogilvie LA, Patel BA, Dedi C, Macfarlane WM, Jones BV. Disruption of Escherichia coli Nissle 1917 K5 capsule biosynthesis, through loss of distinct kfi genes, modulates interaction with intestinal epithelial cells and impact on cell health. PLoS ONE. 2015;10:e0120430.
Sánchez B, Urdaci MC. Extracellular Proteins from Lactobacillus plantarum BMCM12 prevent adhesion of enteropathogens to mucin. Curr Microbiol. 2012;64:592–6.
Yan F, Cao H, Cover TL, Washington MK, Shi Y, Liu L, Chaturvedi R, Peek RM Jr, Wilson KT, Polk DB. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J Clin Invest. 2011;121:2242–53.
Wang Y, Liu L, Moore DJ, Shen X, Peek RM, Acra SA, Li H, Ren X, Polk DB, Yan F. An LGG-derived protein promotes IgA production through upregulation of APRIL expression in intestinal epithelial cells. Mucosal Immunol. 2017;10:373–84.
Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–75.
Shen X, Liu L, Peek RM, Acra SA, Moore DJ, Wilson KT, He F, Polk DB, Yan F. Supplementation of p40, a Lactobacillus rhamnosus GG-derived protein, in early life promotes epidermal growth factor receptor-dependent intestinal development and long-term health outcomes. Mucosal Immunol. 2018;11:1316–28.
Shen X, Liu L, Peek RM, Acra SA, He F, Polk DB, Yan F. P079Neonatal supplementation of a Lactobacillus rhamnosus GG (LGG)-derived protein, P40, promotes intestinal development and prevents colitis in adulthood. Inflamm Bowel Dis. 2018;24:S29.
Seth A, Yan F, Polk DB, Rao RK. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1060–9.
Gao J, Li Y, Wan Y, Hu T, Liu L, Yang S, Gong Z, Zeng Q, Wei Y, Yang W, Zeng Z, He X, Huang SH, Cao H. A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front Microbiol. 2019;10:477.
Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1025–34.
Shimada Y, Kinoshita M, Harada K, Mizutani M, Masahata K, Kayama H, Takeda. Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS ONE. 2013;8:e80604.
Beaumont M, Neyrinck AM, Olivares M, Rodriguez J, de Rocca Serra A, Roumain M, Bindels LB, Cani PD, Evenepoel P, Muccioli GG, Demoulin JB, Delzenne NM. The gut microbiota metabolite indole alleviates liver inflammation in mice. FASEB J. 2018;32:6681–93.
Bansal T, Englert D, Lee J, Hegde M, Wood TK, Jayaraman A. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect Immun. 2007;75:4597–607.
Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci USA. 2010;107:228–33.
Pulakazhi Venu VK, Mahmoud S, Hollenberg MD, Hirota SA. Gut derived indole 3-propionic acid regulates endothelial function in a PXR dependent mechanism. Atheroscler Suppl. 2018;32:125.
Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, Mani S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and toll-like receptor 4. Immunity. 2014;41:296–310.
Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ, Abreu MT. Cox-2 is regulated by toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–77.
Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106:3698–703.
Ehrlich AM, Henrick B, Pacheco A, Taft D, Xu G, Huda N, Lozada-Contreras M, Goodson M, Slupsky C, Mills D, Raybauld H. Bifidobacterium grown on human milk oligosaccharides produce tryptophan metabolite Indole-3-lactic acid that significantly decreases inflammation in intestinal cells in vitro. FASEB J. 2018;32:6681–93.
Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74:81–94.
Lee E-Y, Choi D-Y, Kim D-K, Kim J-W, Park JO, Kim S, et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9:5425–36.
Kang C, Ban M, Choi EJ, Moon HG, Jeon J-S, Kim DK, Park SK, Jeon SG, Roh TY, Myung SJ, Gho YS, Kim JG, Kim YK. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS ONE. 2013;8:e76520.
Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, van der Ark KC, Aalvink S, Martinez LO, Dumas ME, Maiter D, Loumaye A, Hermans MP, Thissen JP, Belzer C, de Vos WM, Cani P. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107–13.
Chelakkot C, Choi Y, Kim D-K, Park HT, Ghim J, Kwon Y, Jeon J, Kim MS, Jee YK, Gho YS, Park HS, Kim YK, Ryu SH. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50:e450.
Anhê FF, Marette A. A microbial protein that alleviates metabolic syndrome. Nat Med. 2017;23:11–2.
Alvarez C-S, Badia J, Bosch M, Giménez R, Baldomà L. Outer membrane vesicles and soluble factors released by probiotic Escherichia coli Nissle 1917 and commensal ECOR63 enhance barrier function by regulating expression of tight junction proteins in intestinal epithelial cell. Front Microbiol. 2016;7:1981.
Hering NA, Richter JF, Fromm A, Wieser A, Hartmann S, Günzel D, Bücker R, Fromm M, Schulzke JD, Troeger H. TcpC protein from E. coli Nissle improves epithelial barrier function involving PKCζ and ERK1/2 signaling in HT-29/B6 cells. Mucosal Immunol. 2014;7:369–78.
Hamer HM, Jonkers D, Venema K, Vanoutvin S, Troost FJ, Brummer RJ. The role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–19.
Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–26.
Elamin EE, Masclee AA, Dekker J, Pieters H-J, Jonkers DM. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J Nutr. 2013;143:1872–81.
Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res. 2007;61:37–41.
Liu H, Wang J, He T, Becker S, Zhang G, Li D, Ma X. Butyrate: a double-edged sword for health? Adv Nutr. 2018;9:21–9.
Burger-van Paassen N, Vincent A, Puiman PJ, van der Sluis M, Bouma J, Boehm G, van Goudoever JB, van Seuningen I, Renes IB. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J. 2009;420:211–9.
VanHook AM. Butyrate benefits the intestinal barrier. Sci Signal. 2015;8:ec135.
Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–47.
Leonel AJ, Alvarez-Leite JI. Butyrate: implications for intestinal function. Curr Opin Clin Nutr Metab Care. 2012;15:474–9.
Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. 2017;52:1–8.
Flemming A. Butyrate boosts microbicidal macrophages. Nat Rev Immunol. 2019;19:135.
Chatterjee I, Kumar A, Anbazhagan AN, Alrefai WA, Borthakur A, Dudeja PK. Butyrate enhances MCT1 association with CD147 via GPR109A activation-dependent mechanisms. FASEB J. 2016;30:1020–3.
Zheng L, Kelly CJ, Battista KD, Schaefer R, Lanis JM, Alexeev EE, Wang RX, Onyiah JC, Kominsky DJ, Colgan SP. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of Claudin-2. J Immunol. 2017;199:2976–84.
Schauber J, Svanholm C, Termén S, Iffland K, Menzel T, Scheppach W, Melcher R, Agerberth B, Lührs H, Gudmundsson GH. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 2003;52:735–41.
Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–71.
Glover LE, Colgan SP. Hypoxia and metabolic factors that influence inflammatory bowel disease pathogenesis. Gastroenterology. 2011;140:1748–55.
Gillor O, Etzion A, Riley MA. The dual role of bacteriocins as anti- and probiotics. Appl Microbiol Biotechnol. 2008;81:591–606.
Heng NCK, Tagg JR. What’s in a name? Class distinction for bacteriocins. Nat Rev Microbiol. 2006;4:160.
Chikindas ML, Weeks R, Drider D, Chistyakov VA, Dicks LM. Functions and emerging applications of bacteriocins. Curr Opin Biotechnol. 2018;49:23–8.
Heng NCK, Wescombe PA, Burton JP, Jack RW, Tagg JR. The diversity of bacteriocins in Gram-positive bacteria. bacteriocins. Berlin: Springer; 2007. p. 45–92.
Alvarez-Sieiro P, Montalbán-López M, Mu D, Kuipers OP. Bacteriocins of lactic acid bacteria: extending the family. Appl Microbiol Biotechnol. 2016;100:2939–51.
Mathur H, Field D, Rea MC, Cotter PD, Hill C, Ross RP. Fighting biofilms with lantibiotics and other groups of bacteriocins. NPJ Biofilms Microbiomes. 2018;4:9.
Pons A-M, Lanneluc I, Cottenceau G, Sable S. New developments in non-post translationally modified microcins. Biochimie. 2002;84:531–7.
Riley MA, Wertz JE. Bacteriocin diversity: ecological and evolutionary perspectives. Biochimie. 2002;84:357–64.
Patzer SI, Baquero MR, Bravo D, Moreno F, Hantke K. The colicin G, H and X determinants encode microcins M and H47, which might utilize the catecholate siderophore receptors FepA, Cir, Fiu and IroN. Microbiology. 2003;149:2557–70.
Bhardwaj A, Gupta H, Kapila S, Kaur G, Vij S, Malik RK. Safety assessment and evaluation of probiotic potential of bacteriocinogenic Enterococcus faecium KH 24 strain under in vitro and in vivo conditions. Int J Food Microbiol. 2010;141:156–64.
Majeed H, Gillor O, Kerr B, Riley MA. Competitive interactions in Escherichia coli populations: the role of bacteriocins. ISME J. 2011;5:71–81.
Cleusix V, Lacroix C, Vollenweider S, Duboux M, Le Blay G. Inhibitory activity spectrum of reuterin produced by Lactobacillus reuteri against intestinal bacteria. BMC Microbiol. 2007;7:101.
Hols P, Ledesma-García L, Gabant P, Mignolet J. Mobilization of microbiota commensals and their bacteriocins for therapeutics. Trends Microbiol. 2019;27:690–702.
Corr SC, Li Y, Riedel CU, O’Toole PW, Hill C, Gahan CGM. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci USA. 2007;104:7617–21.
Graham CE, Cruz MR, Garsin DA, Lorenz MC. Enterococcus faecalis bacteriocin EntV inhibits hyphal morphogenesis, biofilm formation, and virulence of Candida albicans. Proc Natl Acad Sci USA. 2017;114:4507–12.
Kumara SS, Bashisht A, Venkateswaran G, Hariprasad P, Gayathri D. Characterization of novel Lactobacillus fermentum from curd samples of indigenous cows from malnad region, Karnataka, for their aflatoxin B1 binding and probiotic properties. Probiotics Antimicrob. Proteins. 2019;11:1100–9.
Rashmi BS, Gayathri D. Draft genome sequence of the gluten-hydrolyzing bacterium Bacillus subtilis GS 188, isolated from wheat sourdough. Genome Announc. 2017. https://doi.org/10.1128/genomea.00952-17.
Rashmi BS, Gayathri D. Molecular characterization of gluten hydrolysing Bacillus sp. and their efficacy and biotherapeutic potential as probiotics using Caco-2 cell line. J. Appl. Microbiol. 2017;123(3):759–72.
Rashmi BS, Gayathri D. Evaluation and optimization of extracellular digestive enzymes from Bacillus spp. isolated from curds. Matern Pediatr Nutr. 2017;3:1. https://doi.org/10.4172/2472-1182.1000118115.
Gayathri D, Rashmi BS. Mechanism of development of depression and probiotics as adjuvant therapy for its prevention and management. Mental Health Prev. 2017;5:40–51.
Kolb-Mäurer A, Gentschev I, Fries HW, Fiedler F, Bröcker EB, Kämpgen E, Goebel W. Listeria monocytogenes-infected human dendritic cells: uptake and host cell response. Infect Immun. 2000;68:3680–8.
Boneca IG, Dussurget O, Cabanes D, Nahori M-A, Sousa S, Lecuit M, Psylinakis E, Bouriotis V, Hugot JP, Giovannini M, Coyle A, Bertin J, Namane A, Rousselle JC, Cayet N, Prévost MC, Balloy V, Chignard M, Philpott DJ, Cossart P, Girardin SE. A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proc Natl Acad Sci USA. 2007;104:997–1002.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China Program [No. 31820103010, No. 31530056 and No. 31871773]; Wuxi Science and Technology Development Fund Project [CSE31N1429]; National First-Class Discipline Program of Food Science and Technology [JUFSTR20180102]; the BBSRC Newton Fund Joint Centre Award; and Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Author information
Authors and Affiliations
Contributions
QL and ZY contributed to literature search and writing the manuscript. QZ guided the topic of this article and reviewed the manuscript. FT advised the figures and tables. HZ and JZ provided advice and guidance on the relationship between probiotics’ surface substances and intestinal barriers. WC guided the relationship between probiotic metabolites and gut barriers. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Q., Yu, Z., Tian, F. et al. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb Cell Fact 19, 23 (2020). https://doi.org/10.1186/s12934-020-1289-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-020-1289-4