Abstract
Background
The aim was to investigate the prognostic value of MR apparent diffusion coefficients (ADC) using histogram analysis (HA) in predicting disease-free survival (DFS) of cervical cancer after chemo-radiation therapy.
Methods
We retrospectively analyzed 103 women with pathologically proven squamous cell uterine cancer who received chemo-radiation therapy between 2009 and 2013. All patients were followed up for more than 2 years. Pre-treatment MR images were retrieved and imported for HA using an in-house developed software program based on 3D Slicer. Regions of interest of whole tumors were drawn manually on DWI with reference to T2WI. HA features (mean, max, min, 50, 10, 90%, kurtosis, and skewness) were extracted from apparent diffusion coefficient (ADC) maps and compared between the recurrence and non-recurrence groups after the 2-year follow-up. Univariate and multivariate Cox regression analysis was used to correlate ADC HA features and relevant clinical variables (age, grade, maximal diameter of tumor, FIGO stage, SCC-Ag) with DFS.
Results
One hundred three patients with stage IB-IV cervical cancers were followed up for 2.0–94.6 months (median 48.9 months). Twenty patients developed recurrence within 2 years. In the recurrence group, the min (P = 0.001) and 10% (P = 0.048) ADC values were significantly lower than those of the non-recurrence group. Univariate and multivariate Cox regression analysis revealed that ADCmin (P = 0.006, HR = 0.110) was significantly correlated with DFS.
Conclusion
Pre-treatment volumetric ADCmin in histogram analysis is an independent factor that is correlated with DFS in cervical cancer patients treated with chemo-radiation therapy.
Similar content being viewed by others
Background
Uterine cervical cancer is one of the most common malignancies in the female gynecologic system. Due to extremely unbalanced economical and healthcare levels across the world, the incidence and prognosis of cervical cancer vary dramatically among different countries and areas. Most cervical cancer patients in China are at advanced stages when diagnosed. According to the FIGO stage system and NCCN guidelines, locally advanced cervical carcinoma (LACC) usually refers to stage IB2 to IVA cancers, and concurrent chemoradiotherapy (CCRT) is suggested as the standard treatment strategy for such patients [1]. The current concept of cancer treatment calls for personalized treatment in order for the patient to benefit the most and to maximally increase survival time.
Among the factors that are related to the survival rate of cervical cancer, tumor size is the most well-accepted one; it has already been included as a key factor in the FIGO staging system. Other risk factors include positive lymph nodes, parametrial extension, positive surgical margins, lymph vascular space invasion, and depth of invasion [2], but most of this information can only be acquired by operational pathology, which is impossible for LACC patients subjected to radical radiation. Imaging has the ability to provide more information in vivo before treatment, so much research has been carried out on the selection of imaging parameters, such as enhancing patterns, to predict the survival rate of cervical cancer. Comparing with dynamic contrast enhanced parameters, DWI has obvious advantages, such as nonuse of contrast radium, relatively stable image quality, and a universally accepted quantitative index, the apparent diffusion coefficient (ADC).
The results regarding ADC values in predicting treatment effects vary greatly. Some studies showed low ADC values are related with recurrence and a poor survival rate [3, 4], while some found low ADC values in patients with good treatment responses [5, 6]. Other studies concluded that the available evidence is insufficient to use pre-treatment ADC values to predict treatment efficacy [7,8,9]. One of the main disadvantages of ADC measurements is that the location and the sizes of regions of interests (ROIs) may affect the values, which is a common practical problem in almost all parameter measurements. Therefore, the application of histogram analysis (HA) is validated, which generates more indices to describe the distribution of each voxel within ROIs, instead of a single mean value. Studies on HA did achieve inspiring results in predicting cervical cancer survival rates, but large differences exist between studies. One of the possible reasons may still be the choice of ROIs, as some studies used maximum single slices instead of whole tumor slices. We suspected that whole lesion ROIs are able to reflect lesion characteristics to the greatest extent, while small ROIs or single slice ROIs all have the possibility of selection bias. Therefore, in this study, we use whole lesion ROIs and HA to assess the possibility to use ADC values to predict disease-free survival (DFS) of cervical cancer for a follow-up period of 5 years.
Methods
Patients
Informed consents were waived by the institutional Ethics Committee for this retrospective study. All patients with pathologically diagnosed squamous cell uterine cervical cancer in our institute from 2009 to 2013 were reviewed, and 152 consecutive patients were found to meet the following inclusion criteria: (a) the tumor was in stage IB to IVA, (b) the patient received concurrent chemoradiation in our radiation department, (c) pre-treatment pelvic MR was performed in our hospital and was within 30 days before treatment started, and (d) valid follow-up information could be acquired from the medical records. After retrieving MR images and clinical medical records, 49 patients were excluded, including 32 with sagittal instead of axial DWI, 8 with poor quality DWI images, 5 with a tumor with a long diameter of less than 1 cm, 1 with small cell lung cancer, and 3 with a follow-up time of less than 2 years. We recorded the relevant clinical information, including age, grade, FIGO stage, and serum levels of squamous cell carcinoma antigen (SCC-Ag). In total, 103 subjects were enrolled in the study. The follow-up time was defined as from the date of first radiation to either the recurrence date or the last visit date with no events. The median follow-up time for the whole cohort was 48.9 months (range 2.0–94.6 months). Among them, 55 patients free of recurrence or metastasis at the end of the follow-up period (median follow-up time 59.7 months).
Treatment and outcome evaluation
All patients underwent standard radiotherapy in combination with concurrent cisplatin-containing chemotherapy. Radiotherapy consisted of external beam radiation (40–50.4Gy in 1.8-2Gy daily fractions using 10-MV photons) and intracavitary brachytherapy (30–35 Gy to point A by 5 Gy fractions in 3 weeks). Six courses of cisplatin-based chemotherapy (40 mg/m2 dosage) were delivered weekly at the same time with radiotherapy.
The primary outcome was DFS, defined as the period of time from the first radiation treatment to the date of developing any recurrence (local or distant relapse, metastasis). Patients with persistent disease were considered to have relapsed on the first day of completing radiation therapy. Recurrence data were taken from the medical records, with evidence based on the diagnosis by the treating physician and imaging (18F-fluorodeoxyglucose [18F-FDG] positron emission tomography/computed tomography [PET/CT], MRI, CT) or biopsy results. Recurrence group and non-recurrence group were divided based on DFS ≤ 2 year and DFS > 2 years.
MR imaging
All examinations were performed on 1.5-T MR scanners (GE optima 1.5 T MR360 and GE signa 1.5 T EchoSpeed Plus) with four-channel or eight-channel torso phased-array body coil. The scanning range was set to cover the entire pelvis from the level of the anterior superior iliac spine to the inferior level of the symphysis pubis. All patients were required to fast at least 2 h before the examination. After excluding the contradictions, patients were asked to inject 5 ml of anisodamine about 20–30 min before the examination to reduce bowel motion artifacts.
MR sequences included T1WI, T2WI, and DWI. The standard MR scan protocol was kept identical each time and was as follows: (a) axial T1WI: fast spin-echo (FSE) sequence, repetition time (TR) = 726 ms, echo time (TE) = 12 ms, matrix size = 288 × 256, field of view (FOV) = 26–36 cm, slice thickness/intersection gap = 8/1 mm, number of excitations (NEX) = 1; (b) axial T2WI: FSE-XL, TR = 5182 ms, TE = 80 ms, matrix size = 352 × 320, FOV = 26–30 cm, slice thickness/intersection gap = 8/1 mm, NEX = 2; (c) sagittal T2WI: FSE-XL, TR = 4138 ms, TE = 102 ms, matrix size = 320 × 288, FOV = 24–36 cm, slice thickness/intersection gap = 5/1 mm, NEX = 4; (d) axial DWI: single-shot echo-planar imaging sequence, TR = 3500 ms, TE = minimum time, matrix size = 128 × 128, FOV = 36 cm, slice thickness/intersection gap = 8/1 mm, NEX = 4, b-value = 0 and 1000 s/mm2. All slice lines of DWI were copied from the axial T2WI to make sure the images were all at the same table position.
Image analysis
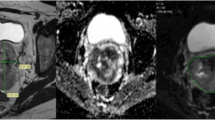
All images were retrieved from local PACS (Huahai, China). By putting axial T2WI and DWI images side by side and also referring to the sagittal T2WI, ROIs were drawn manually slice by slice on DWI images along the edge of the lesions in order to cover as much tumor area as possible without excluding cystic, hemorrhagic or necrotic areas (Fig. 1). ROIs were initially drawn by a junior radiology resident (Z.B.) with 3 years’ experience in MR reading and then reviewed and corrected by a senior attending radiologist (C.K.) with more than 10 years’ experience in gynecologic imaging. Both radiologists were blinded to patients’ outcomes during the data collection period. Whole lesion ROIs were thus achieved to acquire volumetric data of tumors.
Manual segmentation of ROIs in cervical lesion and schematic diagram of parameters. a–c Referring to T2WI and DWI, ROIs were drawn manually slice by slice on DWI images along the edge of the lesions in order to cover as much tumor area as possible without excluding cystic or necrotic areas. d The same ROIs were registered to ADC maps
Axial DWI images were loaded into a post-processing workstation (GE AW 4.6) and parametric ADC maps were generated automatically in the FUNCTOOL program. Parametric maps along with DWI and ROIs were all transferred to house-made radiomics software based on the 3D Slicer platform. ROIs were registered to the parametric maps. Histograms and corresponding ADC parameters values were automatically generated by the software. Excel software was used to sum up the parameters values of ROI at all slices of the tumor respectively and then calculate the average value. The HA program in the software was generated the following 8 parameters for ADC values: mean, median, maximum, minimum, 10th percentile, 90th percentile, kurtosis, and skewness. The maximal diameter of tumor (MDT) was calculated on two-dimensional axial ADC metrics.
Statistical analyses
Continuous variables are presented as mean ± SD. Categorical variables are presented as counts and percentages. The clinical variables and ADC parameter values were compared between the recurrence and non-recurrence groups using the Wilcoxon rank-sum test or the chi-square test. Univariate and multivariate Cox regression analyses were used to evaluate the potential prognostic value of ADC parameters and relevant clinical variables (i.e., age, MDT, grade, FIGO stage, SCC-Ag) for DFS. For the multivariate analysis, parameters were selected by using stepwise selection and by considering the following covariates with a P-value less than 0.3 in the univariate analysis. The receiver operating characteristic (ROC) curve was drawn to determine the cutoff value of the parameters using the maximum Youden index. The Kaplan–Meier survival curve was drawn and log-rank estimates were obtained. All statistical analyses were performed using SPSS 22.0. All reported values are two-tailed, and P-values < 0.05 were considered statistically significant.
Results
Clinical characteristics are listed in Table 1. All patients were followed up for more than 2 years, the median follow-up period was 59.7 months (range, 25.5–94.6 months) for surviving patients. Patient characteristics are listed in Table 1. Of the 103 women analyzed, tumor recurrence was observed in 29 cases (28.2%) at the end of the follow-up period; 11 in the first year, 9 in the second year, and 9 patients suffered from recurrence after more than 2 years of follow-up. Of the 29 patients with tumor recurrence, 3 had persistent cervical cancer, 15 patients showed distant metastasis, and 11 patients showed local relapse in the pelvic cavity. The 1-year and 2-year DFS rates were 89.3 and 80.5%, respectively. Patients characteristics in the recurrence and non-recurrence groups are presented in Table 2. Pre-treatment ADCmin and ADC10% were 0.588 (0.163–5.351) (× 10− 3 mm2/s) and 0.776 (0.543–8.134) (× 10− 3 mm2/s) in the recurrence and non-recurrence groups, respectively.
The values of ADCmin (P = 0.001) and ADC10% (P = 0.048) were significantly lower in the recurrence group than in the non-recurrence group. In the univariate analysis, age, ADCmin, ADC10%, and ADCskewness were associated with DFS (Table 3). In the multivariate Cox analysis, ADCmin of the tumor was the most significant predictor for patient DFS (P = 0.006, HR = 0.110), where a higher ADCmin was significantly associated with poor DFS (Table 3).
ROC analysis of tumor recurrence within 2 years showed that the area under the curve for ADCmin was 0.738 (95% CI, 0.608–0.868; P = 0.001) (Fig. 2). The cutoff value of ADCmin was 0.482 × 10− 3 mm2/sec, with a sensitivity of 84.3% and a specificity of 60%. The DFS for patients with ADCmin ≥ 0.482 mm2/sec was significantly higher than that of patients with ADCmin < 0.482 mm2/sec (P = 0.002, long-rank test) (Fig. 3).
Discussion
Although CCRT is an optimal therapy for LACC with appreciable outcome, treatment for relapse of tumor afterwards remains tough. Thus we assumed it will be of clinical significance to find high-risk patients who subject to recurrence within short time, and who might benefit from additional or novel therapies, such as targeted agents with chemotherapy [10], or adjuvant or consolidation chemotherapy after CCRT [11]. Therefore, functional MR sequences and imaging processing techniques been investigated for this purpose.
In our study, ADCmin and ADC10% were significantly different between the recurrence and non-recurrence groups after 2 years of follow-up, while only ADCmin was found to be correlated with DFS of cervical cancers by multivariate regression analysis. ADC10% and skewness were not associated with tumor recurrence in the multivariate analysis.
In previous studies, ADC values were found to be lower in cervical squamous cell carcinoma compared with adenocarcinoma, even when using HA-derived mean ADCs [3, 12] and minimal ADCs [13, 14]. Therefore, we only included squamous cell cancers in this study, as it is the most common histologic type of cervical cancer and also to exclude possible interference due to different pathology types. ADC values of malignant tumors are commonly lower than those of normal tissues, as is also the case in uterine cervical cancer [3, 15]. However, the usefulness of mean ADC values in predicting therapeutic effects differs between studies, possibly due to inter-observer variations in ROI selection and size. The introduction of HA, by further dissecting and defining the distributions of all voxels involved, helps to provide more information for tumor characterization. Theoretically, only ROIs that cover whole lesions are able to comprehensively reflect tumor characteristics. Even necrosis may represent one of the intrinsic features of a tumor, so we think it is not necessary to exclude the possible necrotic areas, as was usually done in previous studies when using small ROIs.
With the method of HA, lower ADC values were often found to be related to more aggressive and poor prognosis tumors, such as poorly differentiated stage I cervical cancers, compared with well/moderately differentiated ones [16]. In a study by Erbay et al. [17], using HA to achieve several percentile ADC values, they found ADC50%, ADC75%, ADC90%, and ADC95% were lower in patients with tumor recurrence than in those without recurrence. Unfortunately, the percentiles they used were all above 50%. Similar results were also found in a study by Gladwish et al., in which, among different percentiles of ADC values that were higher than 50%, the ADC95% and nADC95% (normalized to urine) were found to be independent protective factors associated with DFS [18]. Another study showed ADC75% to be a risk factor for tumor recurrence [19]. In our study, high percentiles do not seem to be so significant among all histogram parameters, while the lower ADC values (ADCmin and ADC10%) exhibit a difference between the recurrence and non-recurrence groups. Several studies have also shown that ADC10% is significantly associated with event-free survival [20] and DFS [21]. However, in our study, low ADC10% values may be correlated with recurrence within 2 years, yet they are not associated with DFS in our Cox regression analysis; ADCmin is an independent risk factor related to DFS using Cox regression analysis.
Some studies also found that ADCmin is a valuable factor that reflects tumor aggressiveness. The ADCmin of stage T2b-T4 cervical cancers was significantly lower than that of stage T1 to T2a cancers, and it was even lower in patients with lymph node metastasis or distant metastasis [22]. In one histopathologic study, the ADCmin value of cervical cancer exhibited a negative relationship with Ki-67, the index of cellular proliferation (r = − 0.56, CI = − 0.68−− 0.43, [23]). A meta-analysis among various kinds of tumors also found ADCmin had a stronger relationship with Ki-67 compared with ADCmean [24]. In a study on malignant astrocytomas, ADCmin was found to be negatively correlated with the Ki-67 labeling index, which indicates poor prognosis, and the ADCmin value was found to be significantly higher in the stable group than in the progressing group after treatment [25]. Such results confirm the possible application of ADCmin in predicting survival rates; higher ADCmin values may be related to longer DFS, as is also shown in the present study. However, contradictions can also be found in the literature. For example, in a study by Marconi et al. [26], ADCmin was found to be an independent factor related with DFS as well, but it was a risk factor instead of a protective factor. Considering the fact that HA can also be affected by the coverage extent of ROIs, studies with whole tumor ROIs or single slice ROIs may lead to different results. As stated above, we assumed that whole lesion ROIs can reflect intrinsic tumor heterogeneity to the highest extent, so they are more reliable. The different b-values used for DWI scanning form another factor that may affect the results, because there is no single b-value that is uniformly used among studies.
Conclusions
The volumetric ADCmin in HA has the possibility to act as an independent risk factor that is correlated with DFS in cervical cancer patients treated with chemo-radiation therapy. Pre-treatment ADC histogram analysis may identify higher risk of recurrence patients, which will enable greater personalization of cancer care either via a priori treatment decisions or subsequent follow-up regimens.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- CCRT:
-
Concurrent chemoradiotherapy
- DWI:
-
Diffusion weighted images
- FIGO:
-
International Federation of Gynecology and Obstetrics
- HA:
-
Histogram analysis
- LACC:
-
Locally advanced cervical carcinoma
- MDT:
-
Maximal diameter of tumor
- NCCN:
-
National Comprehensive Cancer Network
- OS:
-
Overall survival
- ROI:
-
Regions of interests
- ROC:
-
Receiver operating characteristic
References
NCCN Clinical Practice Guidelines in Oncology - Cervical Cancer. In.; 2017.
Biewenga P, van der Velden J, Mol BW, Stalpers LJ, Schilthuis MS, van der Steeg JW, et al. Prognostic model for survival in patients with early stage cervical cancer. Cancer. 2011;117(4):768–76.
Kuang F, Ren J, Zhong Q, Liyuan F, Huan Y, Chen Z. The value of apparent diffusion coefficient in the assessment of cervical cancer. Eur Radiol. 2013;23(4):1050–8.
Micco M, Vargas HA, Burger IA, Kollmeier MA, Goldman DA, Park KJ, et al. Combined pre-treatment MRI and 18F-FDG PET/CT parameters as prognostic biomarkers in patients with cervical cancer. Eur J Radiol. 2014;83(7):1169–76.
Liu Y, Bai R, Sun H, Liu H, Zhao X, Li Y. Diffusion-weighted imaging in predicting and monitoring the response of uterine cervical cancer to combined chemoradiation. Clin Radiol. 2009;64(11):1067–74.
Himoto Y, Fujimoto K, Kido A, Baba T, Tanaka S, Morisawa N, et al. Pretreatment mean apparent diffusion coefficient is significantly correlated with event-free survival in patients with International Federation of Gynecology and Obstetrics Stage Ib to IIIb cervical cancer. Int J Gynecol Cancer. 2015;25(6):1079–85.
Zhang Y, Chen JY, Xie CM, Mo YX, Liu XW, Liu Y, et al. Diffusion-weighted magnetic resonance imaging for prediction of response of advanced cervical cancer to chemoradiation. J Comput Assist Tomogr. 2011;35(1):102–7.
Rizzo S, Summers P, Raimondi S, Belmonte M, Maniglio M, Landoni F, et al. Diffusion-weighted MR imaging in assessing cervical tumour response to nonsurgical therapy. Radiol Med. 2011;116(5):766–80.
Harry VN, Gilbert FJ, Parkin DE. Predicting the response of advanced cervical and ovarian tumors to therapy. Obstet Gynecol Surv. 2009;64(8):548–60.
Duenas-Gonzalez A, Zarba JJ, Patel F, Alcedo JC, Beslija S, Casanova L, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011;29(13):1678–85.
Yavas G, Yavas C, Sen E, Oner I, Celik C, Ata O. Adjuvant carboplatin and paclitaxel after concurrent cisplatin and radiotherapy in patients with locally advanced cervical cancer. Int J Gynecol Cancer. 2019;29(1):42–7.
Guan Y, Shi H, Chen Y, Liu S, Li W, Jiang Z, et al. Whole-lesion histogram analysis of apparent diffusion coefficient for the assessment of cervical cancer. J Comput Assist Tomogr. 2016;40(2):212–7.
Xue H, Ren C, Yang J, Sun Z, Li S, Jin Z, et al. Histogram analysis of apparent diffusion coefficient for the assessment of local aggressiveness of cervical cancer. Arch Gynecol Obstet. 2014;290(2):341–8.
Liu Y, Ye Z, Sun H, Bai R. Clinical application of diffusion-weighted magnetic resonance imaging in uterine cervical cancer. Int J Gynecol Cancer. 2015;25(6):1073–8.
Chen J, Zhang Y, Liang B, Yang Z. The utility of diffusion-weighted MR imaging in cervical cancer. Eur J Radiol. 2010;74(3):e101–6.
Downey K, Riches SF, Morgan VA, Giles SL, Attygalle AD, Ind TE, et al. Relationship between imaging biomarkers of stage I cervical cancer and poor-prognosis histologic features: quantitative histogram analysis of diffusion-weighted MR images. AJR Am J Roentgenol. 2013;200(2):314–20.
Erbay G, Onal C, Karadeli E, Guler OC, Arica S, Koc Z. Predicting tumor recurrence in patients with cervical carcinoma treated with definitive chemoradiotherapy: value of quantitative histogram analysis on diffusion-weighted MR images. Acta Radiol. 2017;58(4):481–8.
Gladwish A, Milosevic M, Fyles A, Xie J, Halankar J, Metser U, et al. Association of Apparent Diffusion Coefficient with disease recurrence in patients with locally advanced cervical cancer treated with radical chemotherapy and radiation therapy. Radiology. 2016;279(1):158–66.
Heo SH, Shin SS, Kim JW, Lim HS, Jeong YY, Kang WD, et al. Pre-treatment diffusion-weighted MR imaging for predicting tumor recurrence in uterine cervical cancer treated with concurrent chemoradiation: value of histogram analysis of apparent diffusion coefficients. Korean J Radiol. 2013;14(4):616–25.
Ueno Y, Lisbona R, Tamada T, Alaref A, Sugimura K, Reinhold C. Comparison of FDG PET metabolic tumour volume versus ADC histogram: prognostic value of tumour treatment response and survival in patients with locally advanced uterine cervical cancer. Br J Radiol. 2017;90(1075):20170035.
Lin G, Yang LY, Lin YC, Huang YT, Liu FY, Wang CC, et al. Prognostic model based on magnetic resonance imaging, whole-tumour apparent diffusion coefficient values and HPV genotyping for stage IB-IV cervical cancer patients following chemoradiotherapy. Eur Radiol. 2019;29(2):556–65.
Grueneisen J, Schaarschmidt BM, Heubner M, Aktas B, Kinner S, Forsting M, et al. Integrated PET/MRI for whole-body staging of patients with primary cervical cancer: preliminary results. Eur J Nucl Med Mol Imaging. 2015;42(12):1814–24.
Surov A, Meyer HJ, Wienke A. Associations between apparent diffusion coefficient (ADC) and KI 67 in different tumors: a meta-analysis. Part 2: ADCmin. Oncotarget. 2018;9(9):8675–80.
Surov A, Meyer HJ, Schob S, Hohn AK, Bremicker K, Exner M, et al. Parameters of simultaneous 18F-FDG-PET/MRI predict tumor stage and several histopathological features in uterine cervical cancer. Oncotarget. 2017;8(17):28285–96.
Higano S, Yun X, Kumabe T, Watanabe M, Mugikura S, Umetsu A, et al. Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology. 2006;241(3):839–46.
Marconi DG, Fregnani JH, Rossini RR, Netto AK, Lucchesi FR, Tsunoda AT, et al. Pre-treatment MRI minimum apparent diffusion coefficient value is a potential prognostic imaging biomarker in cervical cancer patients treated with definitive chemoradiation. BMC Cancer. 2016;16:556.
Acknowledgements
We thank Hui Liu, Ph.D. for integrating and modifying the radiomics software. Also, we thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This study was funded by Natural Science Foundation of China (grant number 81402167), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support(No.ZYLX201803), Beijing million Talents Project(No.2017A13)and National Key Research and Development Plan(2017YFC1309101, 2017YFC1309104). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
KC and YSS contributed to the conception of the study. XTL performed the data analyses. HTZ contributed significantly to process data. BZ wrote the manuscript. All of the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Clinical Research Ethics Committee of Peking University Cancer Hospital and Institute approved the research and the written informed consent was waived because this was a retrospective and observational study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhao, B., Cao, K., Li, XT. et al. Whole lesion histogram analysis of apparent diffusion coefficients on MRI predicts disease-free survival in locally advanced squamous cell cervical cancer after radical chemo-radiotherapy. BMC Cancer 19, 1115 (2019). https://doi.org/10.1186/s12885-019-6344-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-019-6344-3