Abstract
Background
The exact pathophysiology of cluster headache is unclear. We examined the influence of interneurons on the trigemino-facial reflex arch and the effect of oxygen, by using the nociception specific blink reflex parameters.
Findings
There is no significant effect of oxygen, immediately and over time, on the nociception specific blink reflex parameters in ten male patients during the active phase of cluster headache, outside attacks. Also, there is no significant difference between the symptomatic and asymptomatic side. None of the subjects experienced a cluster headache attack during study participation. We therefore present the collected data as reference values of nociception specific trigeminal stimulation and the effect of oxygen on nociception specific blink reflex parameters.
Conclusion
The nociception specific blink reflex seems not a suitable instrument for exploring the pathophysiology of cluster headache.
Similar content being viewed by others
Introduction
The exact pathophysiology of cluster headache (CH) is unclear. Previous studies have shown that 100% oxygen (O2) therapy is a notable CH attack reliever [1]. Exactly how oxygen exerts its pain reducing effect in patients with CH is uncertain, but it is shown to directly or indirectly cause vasoconstriction. Indirect vasoconstriction can be the result of a possible action on the parasympathetic outflow from the superior salivatory nucleus, as is shown in rats [2].
The blink reflex (BR) is a brainstem reflex, elicited through stimulation of the supraorbital nerve, derived from the first branch of the trigeminal nerve, resulting in a bilateral blink reaction of the eyelids through the facial nerve. The BR is composed of an early pontine response (R1), and a late medullary response (R2) [3]. R1 is oligosynaptic, ipsilateral and not clinically visible, whereas R2 is polysynaptic, bilateral and clinically observable [4]. A nociception specific blink reflex (nBR) can be elicited by transcutaneously selectively stimulating superficial nociceptive A-delta fibers of the supraorbital nerve with a concentric planar stimulating electrode. The response consists of only a bilateral R2. Using the nBR, the function of the afferent trigeminal and efferent facial nerves and their central connections can be assessed [3].
We wanted to examine the influence of interneurons on the trigemino-facial reflex arch and the effect of high-flow (12 liter/minute (L/min)) O2 by using the nBR and its parameters. However, none of the subjects experienced a CH attack during study participation, despite the fact that all of the subjects were in a cluster period at the time. This was possibly due to a preventive effect of nociception specific trigeminal stimulation on CH attacks [5]. We therefore present the data as reference values of the nBR parameters in patients in a cluster period outside a CH attack and the effect of high-flow O2 inhalation.
Methods
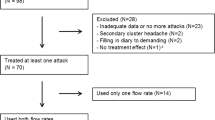
Information concerning study population, in- and exclusion, equipment and questionnaires was already described in a previous publication [5]. The study was approved by the local ethics committee. All patients gave written informed consent. The study terminated early because none of the patients experienced a CH attack during clinical study time. One patient was excluded because the diagnosis of CH was questioned following study participation. CH patients were not compared to healthy controls; the baseline measurement was considered a control.
We elicited nBRs in eleven patients using Synergy EMG equipment (Natus Neurology). For stimulation we used a concentric planar electrode with central cathode and external anode ring (K2 concentric ring stimulating electrode, 1.5 mm; Inomed, Emmendingen, Germany). Disposable silver/silver chloride electrodes were placed over the orbicularis oculi muscles, just lateral of the mid-pupillary line (active) and near the lateral canthus (reference). The ground electrode was placed on the chin. The supraorbital nerve was stimulated ten millimeter cranial of the supraorbital notch with a 200 pulse per second (pps) train of three 0.5 ms pulses. The current intensity was increased stepwise by 0.3 mA, with regard to the tolerance limit of the patient, up to 20 % above the level that acquired stable R2 responses to assure supramaximal stimulation, with a maximum of 2.1 mA (once 2.4 mA). The stimuli were delivered at unpredictable intervals of at least 15 s to minimize habituation. Both the symptomatic and the asymptomatic side were stimulated until we had obtained four blink reflexes on each side (here referred to as one measurement). In each subject, the R2 responses were elicited at at least the five time points: before O2 inhalation, during O2 inhalation and every two hours thereafter up until six hours after O2 inhalation. It was originally planned to continue until a spontaneous CH attack occurred, but this did not happen.
We analyzed the measurements before, during and six hours after O2 inhalation. All responses were evaluated by two researchers (DH and MH). For each stimulation site and time we calculated the shortest latency, amplitude, duration and area of the R2 response using Synergy Reader version 20.1.0.100 (Natus Neurology).
Statistical analysis
We performed the analyses using IBM SPSS statistics version 21. Variables were tested for normal distribution (Shapiro-Wilk). We calculated mean with standard deviation or median with interquartile range as appropriate. Differences of mean were tested with a paired samples t-test. Differences of median were tested using Wilcoxon signed-rank test. Significance levels were adjusted for multiple testing by Bonferroni correction (p < 0.0025).
Results
Ten CH patients were included. All CH patients were men. Mean age was 45.7 (range 24–69). Mean BMI was 24.0 (range 20.5–36.0). Three patients had episodic CH, five patients had chronic CH and two patients were in their first cluster. Six patients experienced attacks on the left side, four on the right. Eight patients were current smokers.
Table 1 shows the nBR parameters of the symptomatic and asymptomatic side after both ipsilateral and contralateral stimulation, and before and during O2 inhalation (n = 10). There is no significant difference in the nBR parameters before and during O2 inhalation. There were also no differences in baseline parameters when the symptomatic side was compared to the asymptomatic side. We then studied the difference between the measurements before O2 inhalation and six hours after O2 inhalation (n = 9; the measurement in one subject was rejected because it was impossible to elicit R2 responses after six hours). This difference was not significant either and we considered the values six hours after O2 inhalation as baseline again.
Discussion
In this study on the pathophysiology of CH using two-hourly transcutaneous stimulation sequences on the supraorbital nerves to elicit the nBR, none of the included patients did experience a CH attack during study participation, This may be an important serendipitous discovery for future prophylactic treatment studies, which we have discussed before [5].
Based on the nBR parameters there is no significant effect of O2, immediately and over time. There is also no significant difference between the symptomatic and asymptomatic side of the nBR parameters during the active phase of CH, but outside CH attacks. The stringent correction for multiple testing poses a risk for false negative results. Using no correction, however, none of the results (except for the ‘ipsilateral shortest R2 latency symptomatic side during O2 administration’ and ‘contralateral area asymptomatic side after six hours’) would have been significant.
It would be interesting to observe what will happen at brainstem level during CH attacks in humans. However, if noninvasive nociception specific supraorbital nerve stimulation (SNS) indeed is confirmed to act in a prophylactic way in CH, it may be difficult to measure nBR parameters during a CH attack.
The nBR was first studied in healthy subjects using a custom built concentric planar stimulating electrode allowing only the nociception specific A-delta fibers to be stimulated [3]. The nBR was further characterized in 104 healthy volunteers without any history of headache. Mean R2 onset latencies were 44.7 ms ipsilateral and 45.4 ms contralateral [6]. We are the first to present nBR reference values in CH patients and the effect of O2 on the nBR parameters. Consequently, it is not possible to make an accurate comparison with other nBR studies.
Our results of the nBR in CH and those from the literature raise some concerns about the applicability of the BR in CH. We searched the literature for BR R2 parameters and found conflicting results with studies indicating no difference between CH patients and healthy controls [7], a decreased excitability in CH patients based on a lower R2 amplitude [8], or an increased excitability based on an increased R2 duration and amplitude [9].
If we combine these variable findings with our own results of the nBR, we feel that the nBR may not be a suitable instrument for exploring the pathophysiology of CH, although a previous BR study suggested otherwise [10]. We have to emphasize that most studies measured conventional non-nociceptive BRs, nevertheless without consistent results. We studied a fairly small homogeneous group of ten male CH patients. It is desirable to study a larger population with both male and female patients comparing CH patients in the active vs the remission phase. Also, the addition of healthy controls is necessary to compare values between groups in further studies.
We conclude that the nBR is not different between symptomatic and asymptomatic sides in patients during the active phase of CH, outside of CH attacks, and that there is no measurable effect of O2 inhalation. Considering our observations with respect to the possible prophylactic action of SNS [5], it is questionable whether it will ever be possible to accurately measure the nBR during CH attacks.
Abbreviations
- BR:
-
Blink reflex
- CH:
-
Cluster headache
- L/min:
-
Liter/minute
- nBR:
-
Nociception specific blink reflex
- O2 :
-
Oxygen
- pps:
-
Pulse per second
- R1:
-
Early pontine response
- R2:
-
Late medullary response
- SNS:
-
Supraorbital nerve stimulation
References
May A, Leone M, Áfra J, Linde M, Sándor PS, Evers S, Goadsby PJ, EFNS Task Force (2006) EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol 13:1066–1077
Akerman S, Holland PR, Lasalandra MP, Goadsby PJ (2009) Oxygen inhibits neuronal activation in the trigeminocervical complex after stimulation of trigeminal autonomic reflex, but not during direct dural activation of trigeminal afferents. Headache 49:1131–1143
Kaube H, Katsarava Z, Käufer T, Diener HC, Ellrich J (2000) A new method to increase nociception specificity of the human blink reflex. Clin Neurophysiol 111:413–416
Aramideh M, Ongerboer De Visser BW, Koelman JHTM, Majoie CBL, Holstege G (1997) The late blink reflex response abnormality due to lesion of the lateral tegmental field. Brain 120:1685–1692
Haane DYP, Koehler PJ (2014) Nociception specific supraorbital nerve stimulation may prevent cluster headache attacks: Serendipity in a blink reflex study. Cephalalgia 34:920–926
Katsarava Z, Ellrich J, Diener HC, Kaube H (2002) Optimized stimulation and recording parameters of human ‘nociception specific’ blink reflex recordings. Clin Neurophysiol 113:1932–1936
Lozza A, Schoenen J, Delwaide PJ (1997) Inhibition of the blink reflex R2 component after supraorbital and index finger stimulations is reduced in cluster headache: An indication for both segmental and suprasegmental dysfunction? Pain 71:81–88
Raudino F (1990) The blink reflex in cluster headache. Headache 30:584–585
Formisano R, Cerbo R, Ricci M, Agostino R, Cesarino F, Cruccu G, Agnoli A (1987) Blink reflex in cluster headache. Cephalalgia 7:353–354
Van Vliet JA, Vein AA, Le Cessie S, Ferrari MD, Van Dijk JG, Dutch RUSSH research group (2002) Reproducibility and feasibility of neurophysiological assessment of the sensory trigeminal system for future application to paroxysmal headaches. Cephalalgia 22:474–481
Acknowledgements
The authors wish to thank J. Haan for his advice on the protocol of the study. This research did not receive a specific grant from any funding agency in the public, commercial or not-for-profit sectors. Materials were supplied by the Department of Neurology and Clinical Neurophysiology, Zuyderland Medical Center Heerlen, Sittard, The Netherlands.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
DH drafted the study protocol, recruited patients, carried out the nBR measurements and helped to draft the manuscript. AP carried out the statistical analysis and drafted the manuscript. PK helped to draft the study protocol, recruited patients and helped to draft the manuscript. MH helped to draft the study protocol, advised on the execution of the nBR measurements, carried out the statistical analysis and helped to draft the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Haane, D.Y.P., Plaum, A., Koehler, P.J. et al. High-flow oxygen therapy in cluster headache patients has no significant effect on nociception specific blink reflex parameters: a pilot study. J Headache Pain 17, 7 (2016). https://doi.org/10.1186/s10194-016-0597-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-016-0597-x