Abstract
Background
Nigeria is endowed with large coal deposits, but some of them are yet to be explored. A combination of proximate analysis and rheological properties were used in characterizing the newly discovered Tai, Garin Maiganga and Shankodi-Jangwa coals in order to determine their suitable applications.
Results
Tai, Garin Maiganga and Shankodi-Jangwa coals were found to have moisture contents of 9.61%, 5.51% and 1.33%; volatile matter contents of 45.11%, 40.53% and 17.37%; ash contents of 23.40%, 20.42% and 17.37%; and fixed carbon contents of 24.11%, 34.75% and 57.57%, respectively. Shankodi-Jangwa coal had the highest percentage of sulphur (1.63%), while both Tai and Garin Maiganga coals had sulphur contents of less than 1%. The agglomerating and rheological properties of the coals revealed that only Shankodi-Jangwa coal has some coking properties with a crucible number of 3.0, temperature range of 107°C, maximum fluidity of 300 and dilatometric G value of 0.96, indicating that it is a low-plasticity and medium-coking coal, which may be blended with strongly coking, low-ash, low-sulphur and high-fluidity coal.
Conclusions
The results indicate that all the coal samples could be suitable for liquefaction; Shankodi-Jangwa coal may be suitable for hydro-gasification in the production of methane. Classification of the Tai, Garin Maiganga and Shankodi-Jangwa coals using the International Standard Organization (ISO) chart revealed that the ISO code numbers are 900, 800 and 622, respectively.
Similar content being viewed by others
Background
Coal is one of the most abundant fossil fuels in Nigeria. Globally, it is one of the leading commodities in terms of industrial importance and monetary value. In Nigeria, there are newly discovered coal deposits, characteristics of which are unknown and are unexplored. Coals are of numerous varieties, and their characterization is very important in deciding their suitable applications. The contemporary global concern for a new source of energy has restored interest in coal. Large coal deposits which have been abandoned due to the availability of petroleum are now being investigated for their potentials in energy generation and other coal conversion processes[1]. The scarcity of prime coking coals has also led to extensive research on blend design and behaviour of blend on carbonization. It is envisaged that if the indigenous coal deposits are explored and exploited, Nigeria's economy can be diversified leading to smooth industrial and technological transitions from the present petroleum-based economy[2].
Rank and type of coal are fundamental factors that control coal characteristics such as the coking properties as well as other numerous technological applications[3]. The effort to source for coking coals, for Nigerian iron and steel industries, has led to the evaluation of coal deposits in different parts of the country; it was discovered that majority of the Nigerian coals are sub-bituminous and lignitic in rank[4, 5]. The coals that were explored and documented are lignites of high volatile matter content, weakly coking coals and medium-coking coals[6]. Therefore, most Nigerian coals are known to be non-coking, but some of them can be blended to obtain blast furnace coke[7–9].
Shankodi-Jangwa coal, Garin Maiganga coal and Tai coal are newly discovered Nigerian coal deposits; their ranks and suitable applications are not well known. The aim of this paper is to use proximate and ultimate analyses and fluidity/plastic properties of the coals in ranking them and in inferring their suitable applications.
Methods
Materials
A total of eight coal samples were obtained from the states of Nasarawa and Gombe, Nigeria. Three samples were collected at different spots from Shankodi-Jangwa coal seam, Obi Local Government Area of Nasarawa State. Three and two coal samples were respectively collected from Garin Maiganga coal mines and Tai coal deposit, both in the Akko Local Government Area of Gombe State. In each case, the coal samples collected from the coal mines were mixed properly and divided into four segments. In each of the segment, a particular size of the sample constituent - large, medium and small particles - was picked round to give almost equal representation of the segment, and the sample at large. A reference coal sample was obtained from the National Metallurgical Development Centre, Jos. The coal samples were shade-dried to remove the free moisture and then ground to fine powder, sieved through 250 and 425 μm, and kept in an airtight polythene bag for further analysis.
Proximate analysis and calorific value determination
The moisture, ash, volatile matter contents and calorific value of the coal samples were determined based on American Society for Testing and Materials (ASTM) D3173, ASTM D3174, ASTM D3175 and ASTM 3286, respectively[10]. The percentage of fixed carbon in the coal samples was obtained by subtracting the percentages of ash, volatile matter and moisture from 100.
Ultimate analysis
Determination of carbon and hydrogen contents
Carbon and hydrogen contents were calculated based on the modified Dulong's formula, i.e. Seyler's formulae[11]:
where Q is the gross calorific value (MJ/kg) and VM is the percentage of volatile matter.
Determination of oxygen content
The oxygen content was obtained by subtracting from 100, the sum of the percentages of carbon, hydrogen, nitrogen and sulphur in the coal sample[11]:
Physico-chemical analysis
Free swelling index determination
A 1.00-g coal sample was weighed into a free swelling index crucible. It was covered, placed over a wire gauge and heated with a Bunsen burner for 7 min. The coke button obtained after cooling was then compared with the standard German profile, numbering from one to nine, increasing in half units.
Gieseler plastometric test
A 5.00 g coal sample, ground to pass a 425 μm sieve, was compacted around the rabble arm of a stirrer in a metal crucible with the help of the preparatory facility. The arm was linked to a motor that provides torque that rotates the stirrer in the compacted coal mass. At the onset, the torque was so small that the stirrer could not rotate when the coal mass was in the solid state. At a point, when the coal mass softened as a result of heat transfer from the lead bath and carbonization temperature increase, fluid was produced as recorded by the rotation of the stirrer which was conveyed via a sensor to the digital output unit, indicating the softening temperature (°C), resolidification temperature (°C), maximum fluidity temperature (°C) and maximum fluidity in dial division per minute (DDPM).
Ruhr dilatometric tests
A 5.00 g coal sample was ground and sieved through 425 μm. The powdered sample was made into a pencil form and was inserted into the dilatometric tubular furnace with a piston weight on top. Then, the setup was gradually heated. The coal mass softened and contracted as a result of the piston weight. With increased softening, more volatile matter trapped within the mass led to correspondent dilatation. The intensity of the dilation was output on a paper. The analytical parameters such as softening temperature (°C), maximum contraction (°C), maximum dilatation temperature (°C), maximum contraction (%) and maximum dilatation (%) were recorded and used in the formula below to obtain the coefficient G, called the coking capacity:
where OE is the initial softening temperature (°C), OV is the final dilatation temperature (°C), c is the percentage contraction and d is the percentage maximum dilatation.
Results and discussion
Proximate analysis
The results of the proximate analysis of the coals and the calorific values are shown in Table 1. The moisture contents range from 1.33% to 9.61%. The Tai coal sample had the highest moisture content of 9.61%, followed by Garin Maiganga and then Shankodi-Jangwa coal samples with 5.51% and 1.33%, respectively. Tai and Shankodi-Jangwa coal samples had the highest and lowest moisture contents of 21.17% and 17.41%, respectively. There is no much difference between the volatile matter contents of Tai and Garin Maiganga coal samples. The Shankodi-Jangwa coal sample had the lowest volatile matter content (29.69%). The highest fixed carbon content of 51.57% was recorded in the Shankodi-Jangwa coal sample, followed by Garin Maiganga and Tai coal samples with values of 34.75% and 24.11%, respectively. The Shankodi-Jangwa coal sample had the highest calorific value of 6,502.70 cal/g, followed by the Garin Maiganga coal sample with 4,981.50 cal/g and then the Tai coal sample with 3,781.50 cal/g.
The moisture content of coal depends on the degree of maturity. The Shankodi-Jangwa coal sample with the lowest moisture content (1.33%) may be the most matured, followed by Garin Maiganga and then Tai coal samples. Moisture content is an indication of the coal's quality; it affects both the calorific value and the concentration of other constituents. High moisture content is disadvantageous because it decreases system capacity and increases operational cost[12]. The moisture content required in a good coking coal is 1.5%[10]. The values recorded for Tai and Garin Maiganga coal samples are generally higher than that stipulated for a good coking coal.
Ash is an inorganic residue remaining after ignition of the combustible fraction. High ash in coal has a negative effect on the strength and performance of coke in the blast furnace as well as in any heating system[13]; it influences slag volume and composition in the blast furnace. The disposal of ash is also a big problem; it increases the operational cost. Industrial experience shows that a 1 wt % increase in ash in the coke reduces metal production by 2 or 3 wt.%. The ash contents in all the coal samples are higher than the 10% specified for a good metallurgical coke making[14].
The volatile matter content of coal depends on its rank. Volatile matter is known to decrease with an increase in rank, and it is one of the most important parameters that determine the suitable application of a coal. In metallurgical coke making, volatile matter does not form part of the coke; it usually evolves as tar during carbonization. High-volatile bituminous coal, due to its high volatile matter content, generates high pressure during carbonization which can damage the coke oven walls[13]. Medium-volatile coals (volatile contents in the range of 27.70% to 30.30%) are known to produce good coke[15]. These suggest that only the Shankodi-Jangwa coal sample, with a volatile matter content of 29.69% (Table 1), falls within the acceptable range for metallurgical coke making.
Fixed carbon, a bituminous material, is the solid residue other than ash obtained by destructive distillation. It is the carbon found in organic materials after volatile materials have been driven off. Fixed carbon determines the rank and quality of a coal sample. High carbon content is essential for coke making coal because it is the mass that forms the actual coke on carbonization[12]. Fixed carbon content is used as an estimate of the amount of coke that will be obtained on carbonization[16]. This suggests that the Shankodi-Jangwa coal sample with the highest value has more carbon for coke formation, followed by Garin Maiganga and then Tai coal samples. The calorific value gives the heating value or the heat of combustion of a substance. It has been suggested that a minimum calorific value of 3,500.00 cal/g may be good for electrical power generation. Thus, all the coal samples may be suitable for power generation. The Shankodi-Jangwa coal sample, with the highest heating value of 6,502.70 cal/g, may be the best for heating and power generation.
Ultimate analysis
The elemental composition of carbon, hydrogen, nitrogen, sulphur and oxygen in the coal samples are shown in Table 2. The elemental compositions with the exception of sulphur were obtained using the modified Dulong's formula, i.e. Seyler's formulae[16]. The percentages of the elemental carbon and hydrogen of Tai, Garin Maiganga and Shankodi-Jangwa coal samples were as follows: 79.25%, 5.58%; 80.24%, 5.27%; and 82.51%, 4.52%, respectively. There is no significant difference between the carbon and hydrogen contents of the coal samples (Table 2). Tai and Garin Maiganga coal samples had low sulphur contents of 0.74% and 0.88%, respectively, while the Shankodi-Jangwa coal sample had the highest value of 1.63%. The oxygen contents of the coal samples fall between 10.03% and 11.95%. The Shankodi-Jangwa coal sample had the lowest oxygen content of 10.03%, followed by Garin Maiganga and Tai coal samples with 11.38% and 11.95%, respectively. The Tai coal sample had the highest nitrogen content of 2.48%, followed by the Garin Maiganga coal sample with 2.23%, while the Shankodi-Jangwa coal sample had the lowest value of 1.31%.
Carbon and hydrogen are the major combustible constituents of coal, and both of them are high in the coal samples (Table 2). The amount of carbon depends on the type of coal, and its percentage increases with rank, from lignite to anthracite. Thus, the carbon content of coal is used in its classification. The higher the carbon content, the higher is the calorific value and the better is the quality of the coal. However, hydrogen is mostly associated with volatile matter, and hence, it affects the use to which the coal would be put to. A good coal sample should have high amount of carbon with low amount of hydrogen, nitrogen and oxygen, as in the case of these coal samples.
Nitrogen and oxygen have no calorific value, but low amount of nitrogen is required in coal because it reduces oxidation. High-oxygen-content coals are characterized by high inherent moisture, low calorific value and low coking power. Moreover, oxygen is found in combination with hydrogen in coal, and thus, hydrogen available for combustion is less than the actual value. A good-quality coal should have low oxygen content, as observed in these coal samples.
Sulphur is one of the major undesirable elements in coal. The Shankodi-Jangwa coal sample had the highest sulphur content of 1.63%, which is above the maximum acceptable limit of 1.5% to 1.60%[17]. High sulphur must be reduced during iron and steel making either by modifying the blast furnace burden with a consequence reduction in iron output or by external desulphurizing technique which are both money- and time-consuming[17]. Even though sulphur contributes to the heating value of coal, on combustion, it produces acids of sulphur dioxide (SO2) and sulphur trioxide (SO3) which corrode the equipment and also cause atmospheric pollution. Sulphur also affects clinkering and slagging tendencies in boilers, corrodes chimneys and other equipment such as air heaters and economizers and limits flue gas exit temperature. The sulphur contents of both Tai and Garin Maiganga coal samples are within the acceptable limit; they may be suitable for smokeless fuel production, heating of residential buildings, firing kiln for cement production, raising steam for power generation and in foundry coke production.
Plastic properties
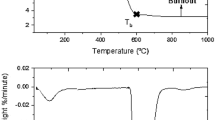
Table 3 shows the results of the free swelling index (FSI) and Ruhr dilatometric properties of the Shankodi-Jangwa coal sample. The results for Tai and Garin Maiganga coal samples are completely omitted because zero crucible numbers were recorded for the FSI and none of the Ruhr dilatometric parameters of the coal samples such as softening temperature, maximum contraction and dilation were detected, so their G values (coking coefficient) are zeros. The Shankodi-Jangwa coal sample had a crucible number of 3.0. FSI is used in assessing the cakability of coals, which is the tendency of coal particles to agglomerate on heating. A coal with a low swelling number will have adequate porosity, while that with a high one will have inadequate strength[18]. FSI is an important parameter used in the International Standard Organization (ISO) classification of coal for coke making. The FSI of zero observed in Tai and Garin Maiganga coal samples shows that they are non-agglomerating and hence non-caking. The crucible number of 3.0 recorded for the Shankodi-Jangwa coal sample shows that it can agglomerate on heating to an extent for coking.
The dilatometric properties of coal are used in assessing its cokability. The parameters are used in calculating the coking capacity (G value). The Shankodi-Jangwa coal sample had average dilatometric properties, softened at a temperature of 380°C, attained a maximum contraction of 19.9% at a temperature of 420°C and then dilated maximally to 9.2% at a temperature of 475°C (Table 3). These values correspond to a G value (coking coefficient) of 0.96. The percentage dilation is also used in the ISO chart for coal classification. The coking coefficient for medium and strongly coking coals lie between Simons' range of 0.95 to 1.15[19]. The G value for the Shankodi-Jangwa coal sample (0.96) places the coal in the medium-coking class, which is also in agreement with the FSI of the coal.
Table 4 shows the results of the Gieseler plastometric analysis of the Shankodi-Jangwa coal sample. The rheological characteristic values like the softening temperature, resolidification temperature and maximum fluidity of Garin Maiganga and Tai coal samples were not detected; therefore, zero values were observed. That is why their values are not shown on the table (Table 4). The Shankodi-Jangwa coal sample was observed to soften at a temperature of 388°C, attained a maximum fluidity of 300 DDPM at a temperature of 455°C and finally resolidified at a temperature of 495°C. The temperature range which is the region between the initial softening and resolidification temperature of coal was 107°C. A coal sample with at least a maximum fluidity value of 300 DDPM may be coking. This test is also significant in the determination of degree of oxidation of a coal as coal plasticity greatly deteriorates with oxidation. In 2002, Diez et al. reported that a Gieseler plastometric maximum fluidity between 200 and 1,000 DDPM is desirable for a good coking coal blend[16].
Plastometry also determines the blendability of two or more coals. Coals can only be blended to form a homogeneous solid coal if their temperature ranges overlap (so that the melting fractions can fuse at almost the same time) and the fluid/plastic levels of the coals balance up. The study of the fluid/plastic behaviour of individual coals is vital in blend formulation. In fact, very few coals by themselves make high-grade blast furnace coke[20]. Therefore, low-fluidity coal can be blended with high-fluidity coal so that the fluid constituent can be combined with the non-melting fractions to form a strong solid mass. The maximum fluidity value of 300 DPPM observed in the Shankodi-Jangwa coal sample indicates that the coal is of medium coking.
ISO classification
Using the ISO classification in which the free swelling index or Roga index determines the group number, the volatile matter (daf) up to 33% and the calorific value (MJ/kg) above 33% of the volatile matter determine the class number, the dilatometric or Gray-King assay parameter determines the sub-group number, the Tai, Garin Maiganga and Shankodi-Jangwa coal samples are of 900, 800 and 622 classes, respectively. The first number indicates the class of the coal, the second number indicates the group of the coal and the third number indicates the sub-group number.
Technological applications
The proximate analysis of the coal samples shows that the Shankodi-Jangwa coal sample had the lowest and acceptable values of volatile matter content (29.69%) and moisture content (1.33%) for metallurgical coke production[21, 22]. Likewise, the Shankodi-Jangwa coal sample had the highest fixed carbon content which would translate into more carbon for coke formation[15]. The crucible number of 3.0 for the Shankodi-Jangwa coal sample shows that it can agglomerate on heating to an extent for coking. Even though the cakability index falls within the range of poor caking coals, the coal is considered to be of some appreciable caking properties which can produce good industrial coke.
The calorific values of all the coal samples are high enough and can be used for power generation. The Shankodi-Jangwa coal sample with the highest heating value of 6,502.70 cal/g may be the best for heating and power generation, but it may require sulphur reduction prior to use. The lower sulphur contents of both Tai and Garin Maiganga coal samples make them more suitable for smokeless fuel production, heating of residential buildings, firing kiln for cement production, raising steam for power generation and in foundry coke production without any concern of sulphur pollution. The coal samples may be used for liquefaction because lignitic, sub-bituminous and high-volatile bituminous coals are potentially good for liquefaction to produce synthetic crude oil and tar[23]. For coal gasification, the Shankodi-Jangwa coal sample, being bituminous, is the only coal sample with potentials for hydro-gasification to produce methane, even though there may be a problem of caking because bituminous coals are known to cake on gasification[23].
Experimental
Determination of total sulphur by the Eschka method
A 1.00 g finely ground (250 μm) coal sample was weighed into a 30-ml porcelain crucible and mixed with 3.00 g of Eschka mixture. The mixture was again covered with 1.00 g of Eschka mixture. The blank and standard samples were also prepared in a similar manner. The crucibles were then placed in a cold muffle furnace and gradually heated to 800°C for about 60 min. The crucibles were then emptied into 400 ml beakers containing 100 ml of hot water. Digestion was carried out for 45 min with occasional stirring. The solution in each beaker was then decanted through a no. 540 filter paper into a 400 ml beaker. Three drops of methyl orange indicator was added dropwise until just neutral. Then, 1 ml of hydrochloric acid was added, after which 25 ml of potassium sulphate solution was also added. The sample was thereafter heated to boiling, and 10 ml of 10% barium chloride solution was slowly added with stirring (to ensure good precipitation). The solution was boiled for 30 min and allowed to cool down to ambient temperature. The solution was then filtered with no. 42 filter paper, and the trapped residue was washed thoroughly with hot water. The filter paper with the residue was thereafter placed into a crucible and smoked off gradually, avoiding burning. The temperature was raised gradually to 800°C and maintained for 60 min. The crucible was then cooled in a desiccator, and the weight of barium sulphate was determined.
The total sulphur content was calculated using the following formula:
where St is the total sulphur, A is the mass of barium sulphate from the sample, B is the mass of barium sulphate from the blank and C is the mass of sample used.
Determination of nitrogen content by the Kjeldahl method
A 2.00-g coal sample was weighed into a Kjeldahl digestion flask. Sulphuric acid (20 ml) and 1 g each of copper sulphate and potassium sulphate (catalysts) were added into the flask. The flask was tilted to about 45°, heated gently until effervescence stopped and then brought to boil. The mixture was then diluted with 100 ml of distilled water and allowed to cool. Thereafter, the flask was connected to a Kjeldahl distillation apparatus, and excess of 50% of sodium hydroxide solution was added to the mixture and then brought to boil. The ammonia gas was condensed into the receiving flask containing 2% boric acid. Bromocresol green and methyl red indicators were added, and the alkaline distillate was titrated against 0.1 M hydrochloric acid. The same procedure was repeated for the blank, and the percentage of nitrogen (% N) was calculated using the formula:
where V 1 and V 2 are the volumes of the hydrochloric acid (ml) used in the sample and the blank, respectively.
Conclusions
This study revealed that Shankodi-Jangwa coal is bituminous in rank, Garin Maiganga coal is sub-bituminous, and Tai coal is lignitic. The maximum fluidity (DDPM), G value and free swelling index (crucible number) indicate that the Shankodi-Jangwa coal sample is of medium-coking class. The high ash and sulphur contents of the Shankodi-Jangwa coal sample makes it suitable for blending with strongly coking and caking, low-ash, low-sulphur and high-fluidity coal. The study revealed that all the coal samples may be suitable for liquefaction and gasification and Garin Maiganga and Tai coals may be used for smokeless fuel production. Based on the ISO classification, Tai, Garin Maiganga and Shankodi-Jangwa coal samples are classified as 900, 800 and 622, respectively.
References
Oshinowo T, Ofi O: Chemical desulphurization of Lafia-Obi coal in aqueous ferric chloride. J Niger Soc Chem Eng 1984,3(1):148–150.
Famuboni AD: Maximizing exploration of Nigeria’s coal reserves. Steel Raw Materials Exploration Agency, Kaduna; 1988.
Jefford G, Cassey OP, de Swardt AMJ: The coal resources of Nigeria. Bulletin No. 28. Federal Government of Nigeria, Lagos; 1963.
Nwadinigwe CA, Egereonu UU: Chemical and instrumental analysis of Enugu coal. Niger J Min Geol 1985, 22: 149–151.
Jauro A, Agho MO, Abayeh OJ, Obaje NG, Abubakar MB: Petrographic studies and coking properties of Lamza, Chikila and Lafia-Obi of the Benue Trough. J Min Geol 2008, 44: 11–18.
Aderonpe WIA: Desulphurization of Nigerian Lafia-Obi coal for metallurgical coke production. PhD thesis. University of Ibadan; 1988.
Afonja AA: A study of potential of Nigerian coals for metallurgical coking. Paper presented at the 1st National Conference on Steel, Ovwian-Aladja, April 1983. 1983, 51–95.
Afonja AA: Optimization of the utilization of sub-bituminous coal in conventional and form coking. Int J Fuel Process Technol 1998, 7: 293–310.
Jauro A, Chukwu CJ: Production of formed coal from Nigerian coals. J Petrol Coal 2011, 53: 1–4.
American Society for Testing and Material (ASTM): Annual book of ASTM standard: petroleum products, lubricants and fossil fuels. ASTM, Easton; 1992.
Francis W, Peters MC (Eds): Fuels and fuels technology. Pergamon, New York; 1980.
Jauro A: Organic geochemistry of Benue trough coals: biomarkers, hydrocarbon generation and coking potentials. LAP Lambert Academic Publishing, Saarbrucken; 2011.
Obaje NG: Petrographic evaluation of the coking potentials of the Cretaceous Lafia-Obi coal deposits in the Benue trough of Nigeria. J Min Geol 1997, 43: 103–176.
Bustin RM, Cameron AR, Greve DA, Kalkreuth WD: Coal petrology: its principles, methods and applications (short course notes). Geological Association of Canada, St. John's; 1985.
Adeleke AA, Onumanyi P, Ibitoye SA: Mathematical optimization of non-coking coal inclusion in coking blend formulations. J Petrol Coal 2011, 53: 212–217.
Diez MA, Alvarez R, Barriocanal C: Coal for metallurgical coke production: predictions of coke quality and future requirements for coke making. Int J Coal Geol 2002, 50: 389–412. 10.1016/S0166-5162(02)00123-4
Mason DM, Gandhi K: Formulas for calculating the heating value of coal and coal char: development, tests and uses. Am Chem Soc Div of Fuel Chem 1980, 25: 235–245.
Williams K: Primary coal - analytical needs. J Pure Appl Chem 1977, 49: 1465–1473. 10.1351/pac197749101465
Nasirudeen MB, Jauro A: Quality of some Nigerian coals as blending stock in metallurgical coke production. J Miner Mater Char Eng 2011, 10: 101–109.
Adeleke AO, Olulana AO, Adahama AB, Makan RS, Ibitoye SA: A prediction of micum strength of metallurgical coke using Ruhr dilatometric parameters of parent coals. J Petrol Coal 2009, 51: 75–79.
Ahindra G, Amit C: Theory and practice of iron and steel making. Prentice-Hall, New Delhi; 2008.
Walker R, Mastalerz M, Padgett P: Quality of selected coal seams from Indiana: implications for carbonization. Int J Coal Geol 2001, 47: 277–288. 10.1016/S0166-5162(01)00046-5
James GS: The chemistry and technology of coal. 3rd edition. CRC/Taylor & Francis, Boca Raton; 2012.
Acknowledgements
The authors wish to acknowledge the Metallurgical Development Centre (NMDC), Jos, for granting a study fellowship to the first author and for allowing the use of their laboratory facilities. Abubakar Tafawa Balewa University, Bauchi, especially the staff of the Chemistry Programme, are well acknowledged for all their support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SAR conducted all the experiments mentioned in the manuscript. AJ participated in the design of the study and in drafting the manuscript. Both authors have read and approved the manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ryemshak, S.A., Jauro, A. Proximate analysis, rheological properties and technological applications of some Nigerian coals. Int J Ind Chem 4, 7 (2013). https://doi.org/10.1186/2228-5547-4-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5547-4-7