Abstract
Background
Invasive (IPD, defined as detection of pneumococci in sterile body fluids like meningitis or bacteremic pneumonia) and non-invasive Streptococcus pneumoniae infections (i.e. non-bacteremic pneumonia, otitis media) in adults are associated with substantial morbidity, mortality and costs. In Germany, Pneumococcal polysaccharide vaccination (PPV23) is recommended for all persons >60 years and for defined risk groups (age 5–59). The aim of this model was to estimate the potential cost-effectiveness and benefit-cost ratios of the adult vaccination program (18 years and older), considering the launch of the pneumococcal conjugate vaccine for adults (PCV13).
Methods
A cross-sectional steady state Markov model was developed to estimate the outcomes of PCV13, PPV23 vaccination schemes and ‘no vaccination’. Conservative assumptions were made if no data were available for PCV13 and PPV23 respectively. The effectiveness of individual pneumococcal vaccination in adults was adjusted for expected indirect effects due to the vaccination in infants. Data on incidences, effectiveness and costs were derived from scientific literature and publicly available databases. All resources used are indicated. Benefit-cost ratios and cost-effectiveness were evaluated from the perspective of the German Statutory Health Insurance as well as from social perspective.
Results
Under the assumption that PCV13 has a comparable effectiveness to PCV7, a vaccination program with PCV13 revealed the potential to avoid a greater number of yearly cases and deaths in IPD and pneumonia in Germany compared to PPV23. For PCV13, the costs were shown to be overcompensated by monetary savings resulting from reduction in the use of health care services. These results would render the switch from PPV23 to PCV13 as a dominant strategy compared to PPV23 and ‘no vaccination’. Given the correctness of the underlying assumptions every Euro spent on the PCV13 vaccination scheme yields savings of 2.09 € (social perspective: 2.16 €) compared to PPV23 and 1.27 € (social perspective: 1.32 €) compared to ‘no vaccination’, respectively.
Conclusions
Results of the model indicate that the health economic benefit of immunizing adults with PCV13 can be expected to outperform the sole use of PPV23, if the effectiveness of PCV13 is comparable to the effectiveness of PCV7.
Similar content being viewed by others
Background
Streptococcus pneumoniae (pneumococcus) is worldwide a leading cause of infections associated with high case fatality rates like sepsis, meningitis and pneumonia. Of the 91 identified pneumococcal serotypes, 10 to 15 pose major risks to morbidity and mortality, particularly in young children (age ≤5 years (y)), elderly (age ≥65 y) and immunocompromised patients (all age groups). In addition, pneumococcus is the most common cause of community-acquired pneumonia (CAP) in adults.
In 2001, a 7-valent pneumococcal conjugate vaccine (PCV7) became available in Europe for children. Higher-valent PCVs were launched in 2009, a 10-valent (PCV-10) and a 13-valent pneumococcal conjugate vaccine (PCV13). Since August 2006 the Standing Vaccination Committee at the German Robert-Koch Institute (STIKO) recommends vaccination of all children less than two years of age. In addition, children at risk should be vaccinated between the ages of 3 and 5 years, since August 2010 with the 13-valent pneumococcal conjugate vaccine. The conjugate vaccine induces a strong antibody response in children and reduced significantly the incidence of IPD in Germany [1] and other European countries.
Contrary to the recommendations for children, the 23-valent pneumococcal polysaccharide vaccine (PPV23) is still the recommended vaccine in Germany for prevention of pneumococcal diseases in elderly (age ≥60 y) and adults at risk despite the ongoing controversy regarding its effectiveness. A recently published meta-analysis and a series of published studies did not confirm the effectiveness of PPV23 and concluded that policy-makers should reconsider their current recommendations for the use of the pneumococcal polysaccharide vaccine, especially when pneumococcal conjugate vaccine get licensed for adults [2–12].
The T-cell dependent immune response of PCV13 induces antibody titres in elderly comparable to those induced in infants. [13] Vaccination studies have shown that antibody levels after PCV13 were at least similar or superior for most vaccine shared serotypes to that induced by PPV23. However, implementing a PCV13 vaccination scheme in adults will cause additional costs in the German health care system due to the higher price of the vaccine. Therefore, this analysis aims to evaluate the benefit-cost ratio and cost-effectiveness of an adult vaccination program in individuals older than 50 years compared to the existing PPV23 recommendation for adults as well as to ‘no vaccination’.
Methods
Model structure
To compare the benefit-cost ratio and cost-effectiveness of three different pneumococcal vaccination strategies (PPV23, PCV13 and ‘no vaccination’) in adults, we updated and extended a recently developed Markov model. The analysis focused on a comparison between a PCV13 and a PPV23 vaccination strategy. Therefore, we simulated a setting in which both vaccination programs were fully established according to the vaccination recommendations in Germany. For the following, we define this setting, in which all new entrants of the target groups were being vaccinated or re-vaccinated and all other individuals were already immunized according to the vaccination recommendations and assumed vaccination rates, as a steady state. Hence, time series of starting and establishing a vaccination were not included in the analysis. The evaluation took the perspective of the German Statutory Health Insurance (SHI), and additionally estimated social work loss costs. Outcomes and costs of each vaccination strategy for one year in a steady state were calculated in two steps.
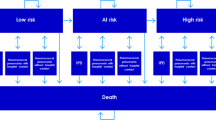
Due to a lack of epidemiological data, we estimated the population at risk (distinguishing between normal, moderate and high risk of pneumococcal disease) in 83 age groups (18–100 years of age) in the above mentioned setting as well as the proportion of immunized individuals due to vaccination in each age/risk group using a Markov state transition model with a time horizon of 100 years. The cycle length was one year. In particular, the model took account for effects of immunized individuals transiting to other risk groups over time. The population simulation started with healthy and unvaccinated newborns developing age-specific risk of comorbidities with increasing age which are associated with moderate or high risk of pneumococcal disease. Each risk group got vaccinated according to the strategies described below. Group members at moderate or high risk remained in their risk group during the simulation. Transition to death was possible from all states. In order to account for the age distribution of the German population, each age group was weighted according to the German population structure in the year 2008 [14].
Secondly, for the modelling of cost-effectiveness number of cases and deaths per year due to pneumococcal diseases were estimated for each vaccination strategy based on the results of the simulated population. To avoid interferences with the recently published children model, children and adolescents were excluded. Risk and age specific morbidity and mortality rates as well as the effectiveness of the vaccination were taken into account. In addition, the risk model calculated the yearly costs of the vaccination programs and yearly treatment costs for pneumococcal diseases.
The time horizon of one year avoided discounting, a topic of high relevance and unclear positioning in the indication of prevention. On the other hand, the cross sectional design of the model neglected costs associated with the implementation of a new vaccine combined with sunk costs of a new catch-up vaccination, which might be of interest regarding the budget impact of a new vaccination program.
The model was constructed in Microsoft Excel 2007.
Vaccination strategies/choice of comparators
In Germany PPV23 vaccination is generally recommended for adults ≥60 y and for all patients >5 y with comorbidities and increased risk of pneumococcal disease. Since 2009, revaccination every 5 years is restricted to patients with immuno-compromising conditions due to frequent adverse events at the site of injection and questionable effectiveness of the vaccine [15].
To estimate the effects of PCV13 vaccination, a hypothetical vaccination program with vaccinating adults older than 50 years (branch 1 = PCV13) was compared to the existing PPV23 recommendation (branch 2 = PPV23). In the PCV13 branch, we assumed that adults at risk were vaccinated with PPV23 until they reached the age of 50. Thereafter they switched to PCV13 when they were revaccinated. That goes in line with current recommendation for pneumococcal vaccination and the targeted label for PCV13. Adults developing comorbidities associated with moderate or high risk of pneumococcal disease after the age of 50 were initially and revaccinated with PCV13 as were seniors with normal risk who got their initial vaccination at the age of 60, analogous to the PPV23 recommendation. For PCV13 the need for a revaccination in adults has not been established. Nevertheless we assumed a decennial booster, based on the experience of pneumococcal conjugate vaccination in children [16–18].
Due to the lack of data, we had to assume vaccination rates for initial and booster vaccination. Considering that the population at risk gets a higher awareness, initial vaccination rates were assumed to be higher than in the risk free population (40% vs. 25%). PPV23 booster vaccination was only considered for every patient in the high risk groups according to STIKO recommendations [15, 19]. In contrast, PCV13 booster vaccination was considered for every patient at risk and every second without any risk. Due to the assumed superior effectiveness of PCV13 restrictions regarding revaccination are not expected.
In a third branch we modelled the ‘no vaccination’ scenario representing the strategy not to prevent but to treat pneumococcal disease. We included this scenario to analyse the total effects of both vaccines in addition to the incremental effects of PCV13 versus PPV23.
Epidemiology
The simulated model population (see Figure 1, population simulation model) size was around 82,000,000 individuals representing the estimated German population of adults in 2008. [14] The age structure of the initial cohort and the number of patients at risk, estimated according to data of Fleming et al., are shown in Table 1. Calculation of life expectancy was based on the German life tables from 2007/2009 [20], while the population structure was taken from the German Federal Statistical Office (2008) [14]. In 2008, the overall German population of 82.0 million continued to decrease. Following the trend of recent years the age group >60 y continues to grow. To model the cost-effectiveness, a population size of 69,200,000 Mio was used (age 18–100), considering different risk groups.
In contrast to the health economic endpoint, the epidemiological endpoints are avoidable episodes of IPD and non-bacteremic CAP in an inpatient setting as well as in an outpatient setting. Historical incidences for IPD in adults are taken from a regional surveillance study in North-Rhine Westphalia prior to the general PCV vaccination recommendation for children. The age-specific IPD incidence was adjusted for underreporting with a factor of 2.7, due to the very low standard with regard to the collection of blood cultures in Germany.
The burden of pneumococcal pneumonia is high and the most serious complication is a bacteremic course associated with a high case fatality rate. The CAP incidence incorporated all causes as detailed microbiological diagnosis is not performed on a regular basis. Data on incidence of CAP treated in hospital (“inpatient CAP”) are based on the German hospital admission data (2008). [22] The BQS-Report documented that 200,000 CAP episodes were treated within hospital every year, which is very close to the numbers incorporated in our model based on admission data. [23] Age specific incidence of CAP treated in primary care settings (“outpatient CAP”) were derived from a representative pharmaceutical prescription panel which results (12 episodes in 1,000 adults) were similar to results reported in the literature. [24] For Schleswig-Holstein (a Northern region in Germany), incidence of CAP ranged between 3.7 and 12.3 cases per 1,000 adults.
Due to the lack of valid data, odds ratios for IPD and CAP incidences in moderate and high risk population were assumed to be 1.5.
The IPD case fatality rate (CFR) for adults was taken from the literature (US 2000–2004). In comparison, the German CFR due to streptococcal and other sepsis cases (ICD A40, A41, A49.1 and G00.1) in the age groups <15, 15 - < 45, 45 - < 65, 65 - < 75, 75 - < 85 and ≥85 was 1.20%, 3.56%, 7.23%, 9.63%, 14.93%, 21.24%. [22] The study of Dzupova et al. confirmed a CFR for adult pneumococcal meningitis of 20%.
The 6 months CFR of inpatient CAP was based on data from the German CAPNetz [25]. While the CFR in CAP in outpatient settings amounted to about 1%, CFR in inpatient CAP was higher (see Table 2). CFR for outpatient CAP was assumed to be 0% for patients <60 y and 0.5% for patients being 60y and older.
Effectiveness
The effectiveness of different pneumococcal vaccines depends on immunization level, vaccine efficacy in invasive and non-invasive pneumococcal diseases differentiated to age and risk groups, serotype coverage, vaccine effectiveness against cross-reactive serotype, and duration of protection, replacement, revaccination, indirect (herd) effects in the same target group, and indirect (herd) effects from vaccination of children. Data for vaccine efficacy and effectiveness are shown in Table 3.
According to published data and adaption of general recommendation, we calculated IPD effectiveness of both vaccines using efficacy data, adjusted to serotype distribution in Germany for all age groups.
For PPV23, IPD data were derived from a Cochrane meta-analysis [9] reporting an correlate IPD efficacy of 74% (with no present statistical heterogeneity when all RCT´s were considered). However, the reported correlate IPD efficacy was not representative for subgroups e.g. population with chronic illness in high income countries. With a 95% CI efficacy ranges overall from 56% to 85%. So we used the upper and lower bound for sensitivity analysis. In contrast, the reported efficacy data for CAP were inconclusive with substantial statistical heterogeneity [9]. Incorporating the findings from a Canadian meta-analysis [6] the conclusion can be drawn, that PPV23 does not appear to be effective in preventing CAP. Therefore, we assumed in the base case analysis that PPV23 is ineffective in preventing CAP. We abandoned this assumption in the sensitivity analysis by calculating the economical outcomes of PPV23 effectiveness against inpatient CAP according to Maruyama et al. [29].
For PCV13, assumption on IPD efficacy was based on clinical data for PCV7 in children for all patients, expecting similar levels of efficacy against the additional 6 serotypes which are not included in PCV7. Serotype coverage was calculated on the basis of data reported by Reinert et al. [30] Effectiveness of initial vaccination on inpatient and outpatient CAP for PCV13 was assumed to be 26% and 6%, respectively, according to effectiveness data on PCV7 [27, 28].
We assumed that vaccines were equally effective against all vaccine serotypes, and further assumed that effectiveness of revaccination with PCV13 and PPV23 was the same as that of initial vaccination. Immunization due to vaccination with PCV13 and PPV23 was assumed to be effective for 10 years and 5 years, respectively. Then the effects wane completely.
In our model, we assumed that childhood vaccination programs with PCV7 were already established. Since children vaccinated with a conjugated vaccine are unlikely to be carriers for the seven vaccine serotypes, they can no longer transmit these to others. In particular, elderly benefit from this indirect protection. Vaccinated children, vaccinated successfully, are no carrier of pathogens. Indirect effects reduced probability of infection in the elderly. Evidence for indirect effects was reported from the US and Australia [31–36].
Markov cohort models are not able to directly capture indirect herd effects based on transmission dynamics of infectious disease. Hence, we included one additional parameter “herd effects” in our model. To address indirect effects from the childhood vaccination, the effectiveness of adult vaccination was reduced by a factor of “herd effects”. The disease and age group specific values for this correction factor are shown in Table 4. The figures were calculated based on US data from Ray et al. [34] and Pilishvili et al. [37] adjusted for German serotype coverage according to Claes et al. [38]. Effects due to serotype replacement were not included. In contrast to the childhood PCV vaccination, adults receive just one dose of vaccine which is expected to be insufficient to induce any herd effects. In addition, PPV23 is unable to elicit immune memory, so that also a second dose of vaccine would not boost antibody level and therefore would not cause significant herd effects [39]. Hence, herd effects due to adult vaccination were not included in the model.
Economic parameters
Life years gained (LYG) were the primary health-economic endpoint in our analysis. Prices and utilization of health services per unit were modeled separately. We evaluated the resource use from the perspective of the SHI in Germany, taking into consideration patient co-payments as well as discounts for medications given by the manufacturer and pharmacies as required by legal obligations in Germany. In addition, we estimated costs from a social perspective, including costs for work loss and patients co-payments. To evaluate the costs from the perspective of the SHI, the current German guidelines for the valuation of resource usage were applied. Costs referred to the year 2010.
Costs of medical care of pneumococcal associated infections
In the German health care system, inpatient treatment is reimbursed by G-DRG and outpatient treatment by official German Uniform Valuation Scheme (EBM). Table 5 summarizes the costs, which were integrated into the model. We assumed that all IPD cases were treated in hospitals due to the severity of invasive infection. Further we assumed that 20% of all inpatient CAP cases took a more severe course. No cost for long-term disabilities from IPD and CAP were included.
Inpatient costs were derived from official G-DRGs codes (German Diagnosis Related Groups) in 2010 which are calculated based on resource use in 2008 (see Table 5). [40] These costs are lump sums and cover all cost per case which includes, in general, all expenses of the hospital (incl. medication costs). The G-DRG system separately covers the specific need for mechanical ventilation. According to this we assumed 13% [41] and 11% [42] cases with mechanical ventilation for IPD and moderate inpatient CAP respectively. For severe inpatient CAP the DRG code E77A covers cases with a high degree on co-morbidities and complex intensive care treatment.
Regarding the outpatient setting, we applied the official German Uniform Valuation Scheme (EBM). [43] For general outpatient physician visits of SHI-insured persons the doctors are reimbursed via capitation fees per quarter, independent from the number of visits of a patient per quarter. The costs of outpatient care consisted of a quarterly capitation fee in 2010: 6y–59y 30.84 € (EBM 03111: 880*0.035048) > 60 y 35.74 € (EBM 03112: 1020*0.035048) plus an average compensation for prescriptions of 23.91 € per episode generated from the IMS prescription panel [24, 43]. The average compensation was calculated on the basis of prescription costs and prescription quantity of ICD10 diagnosis (J12–J18). No extra costs for treating adverse events from vaccination were considered in the model.
Costs of the vaccination program
Based on official data and a package size of ten [44] the pharmacy retail price of PCV13 was determined to be 64.62 € per dose PCV13 and 28.94 € per dose for PPV23. There is no official rule for vaccine pricing in Germany. Therefore no manufacturer and pharmacy discounts were subtracted from the pharmacy retail prices for vaccines. Based on a sample of German vaccination agreements between the Association of SHI Physicians and the SHI we calculated an average reimbursement fee of 6.95 € for each injection. It was assumed that the vaccine is administered at the same time as other vaccines so no further visit costs are incurred.
Indirect costs
Regarding the social perspective cost of work loss and patient’s co-payments were included in the analysis. The cost of work loss was calculated using the human capital approach as proposed by the “Hannoveraner Konsens” [45]. Work loss was expected by an average of 7.4–18.7 days. [46] Assuming an age specific labor participation of ∅ 81.8% for men and ∅ 69.6% for women, daily cost for work disability of 95.72 € were considered as indirect cost in the model [46, 47].
Sensitivity analysis
To access the impact of various parameters on model outcomes and to confirm the robustness of the model, all parameter values were varied individually in one-way sensitivity analysis (SA) and simultaneously in a probabilistic SA with 10.000 iterations. For probabilistic SA, beta distributions were assumed based on published 95% confidence intervals. If confidence intervals or ranges were not reported in the studies, we assumed an upper and lower limit of ±25% of the base case value. To account for skewness in cost data we used gamma distributions. Means and standard errors were derived from the corresponding databases (DRG Browser [40], IMS prescription panel [24]). Means, standard errors, and distributions for all parameters examined in the probabilistic sensitivity analysis are shown in the Appendix.
Results
Estimated effects on IPD and CAP according to the different vaccination strategies are shown in Table 6. Maintaining vaccination with PPV23, 916,233 episodes and 38,967 deaths related to pneumococcus-induced diseases could be expected each year, which is a reduction of 564 episodes and 97 deaths in comparison to ‘no vaccination’ against pneumococcal diseases. Assuming the PCV7 effectiveness data, with PCV13 vaccination (vaccination in adults older than 50 years) 19,009 episodes and 2,661 deaths could be avoided in comparison to ‘no vaccination’.
The results of the base case analysis (Table 7) indicated that switching to PCV13 in adults older than 50 years was cost-effective compared to PPV23 as well as to ‘no vaccination’ and dominated both strategies. According to our model, 22,942 additional life years can be gained by offering PCV13 instead of PPV23 and 24,480 life years can be saved by the PCV13 vaccination program compared to the ‘no vaccination’ scenario. The estimated benefit-cost ratio of PCV13 was 2.09 (2.16 including indirect costs) compared to PPV23 and 1.27 (1.32 including indirect costs) compared to ‘no vaccination’, respectively. Hence, additional cost savings via avoided health care services overcompensated costs related to PCV13 vaccination in adults aged >50 y. The main driver is the prevention of inpatient CAP cases accounting for 80 to 90% of the overall monetary savings of PCV13 over PPV23 and ‘no vaccination’, respectively.
Figure 2 shows results of the one way SA when the same variation factor (+/−25%) was assumed. Overall, the higher the PCV13 price as well as the indirect herd effects, the lower the benefit-cost ratios. Otherwise the higher the incidence rates, the PCV13 effectiveness as well as the medical costs the higher the benefit-cost ratio. Results of one way SA were highly sensitive to variations of the PCV13 price as well as all parameters of inpatient CAP and less sensitive to variations of vaccination rates, indirect herd effects as well as all parameters related to IPD. Variations of parameters related to outpatient CAP had no impact. Lower PCV13 vaccination rates in the normal risk group increased the benefit-cost ratio.
In addition, we tested extreme scenarios, e. g. revaccination frequency of PCV13 = 5 years, no indirect herd-effects through PCV7 vaccination in children, PCV13 completely ineffective in preventing CAP (see Table 8). Even if the monetary net benefit of the PCV13 strategy compared to PPV23 vaccination was negative in some of these scenarios, PCV13 vaccination would be a cost-effective option according to a ICER threshold (assumed between 20,000 and 50,000 €/LYG). Regarding the Maruyama et al. [29] scenario (PPV23 inpatient CAP efficacy = 45%) the PCV13 vaccination program would still be cost-effective with an ICER of 590 €/LYG compared to PPV23, but wouldn’t be a dominant strategy anymore. In a case that PCV13 is completely ineffective in preventing CAP, the ICER of PCV13 compared to PPV23 was 17,983 €/LYG and compared to ‘no vaccination’ 16,440 €/LYG.
Figure 3 and 4 illustrate the results of the probabilistic sensitivity analysis. The minimum benefit-cost ratios of PCV13 in comparison to PPV23 as well as ‘no vaccination’ were 0.62 (0.65 including indirect cost) and 0.41 (0.44 including indirect cost), respectively. PCV13 dominated PPV23 with a probability of 99.51% and ‘no vaccination’ with a probability of 80.46% according to the simulation.
Discussion
This study is the first to describe the benefit-cost ratio and cost-effectiveness of a PCV13 vaccination scheme for adults in Germany. The model found that switching from PPV23 to PCV13 in adults older than 50 years was a dominant strategy. Compared to PPV23 as well as ‘no vaccination’, the PCV13 vaccination program revealed the potential to avoid a greater number of yearly cases and deaths due to the potential superior efficacy of PCV13 in IPD and CAP in Germany. One way sensitivity analyses illustrated PCV13 attained a positive net benefit over PPV23 and over ‘no vaccination’ in most of the tested scenarios and an acceptable ICER in all tested scenarios. Variations in input parameters related to inpatient CAP as well as the price of PCV13 had the most significant impact on the results.
The study was mainly limited by lacking clinical data on conjugate vaccine in adults. Except for a study in HIV-infected adults in Malawia using PCV7, no data are available for an effect of conjugate vaccine in adults. Therefore, data on the vaccine efficacy and effectiveness in infants were used as basis for the assumptions and the fact, that immunogenicity data in adults were comparable to immunogenicity data in children. Initial studies for antibody detection underline these assumptions for PCV7 and PCV13 respectively.
There was a lack of evidence for the duration of protection of PPV23 [9]. Different values were published regarding the PPV23 serotype effectiveness against IPD. Therefore, we decided for the base case scenario to use the recommendations according to the Cochrane meta-analysis [9].
In terms of pneumonia four randomized, placebo-controlled trials of vaccination with PPV23 in COPD (chronic obstructive pulmonary disease) have failed to show a significant reduction in mortality, hospitalization, or pneumonia. However, a recent randomized placebo controlled trial in Japanese nursing home residents (85 +/− 8y) revealed a reduction of all cause pneumonia by 45% and of pneumococcal pneumonia by 64%. The reason for these discrepancies remains unclear.
Since data regarding the waning efficacy of PCV13 vaccine are not available, several publications for PPV23, which value the issue of waning, were considered. For PPV, antibody levels to several serotypes decline to pre-vaccination values within 5–10 years corresponding to a decline of protection. Existing immunogenicity data suggest that PCV will provide a long-lasting immunologic memory and protection. [49, 50] This issue will be addressed in upcoming publications.
Although evidence of indirect (herd) protection for vaccination in children is demonstrated for North Rhine Westphalia, Saxony and Bavaria we decided to use data from U.S, were evidence is confirmed for the whole population. [51] A study by Ardanuy et al. showed evidence of a decrease in IPD due to PCV7 serotypes for hospitalised adults in Spain for adults aged 18–64 (results not significant) and adults over 65 (significant results). [52] As mentioned in section two, we modelled the cost-effectiveness only for adults (≥18 y). To avoid double counting indirect (herd) effects from children vaccination were excluded. Therefore results in terms of case reduction may be underestimated.
Our results were based on the premise that no replacement of serotypes (i.e., an increase in carriage of and disease from serotypes not included in the vaccine) will take place in Germany, though other studies have noted the importance of collecting data on the impact of serotype evolvement on future evaluations of vaccines for pneumococcal disease. This assumption is obviously in contrast to numerous studies showing serotype shifting after introduction if the vaccine and first signs of serotype shifting have already been observed in Germany. [53] However, since the most of the replacement serotypes, particularly the main replacement serotype 19A, is contained in the novel PCV13, a substantial replacement to this vaccine cannot be foreseen. Some authors also assume that replacement serotypes are less fit and virulent compared to the predominant vaccine serotypes before introduction of PCV 7.
In our base case analysis the ICER of PPV23 compared to ‘no vaccination’ was 14,751 €/LYG, which was comparable to results of a published health economic review. [54] This review showed that PPV23 vaccination was a cost-effective option in vaccination over 65 years old mainly in prevention of IPD in most of the studies. Compared to PCV13, vaccination with PPV23 was an inferior strategy in our model. That goes in line with a recently published study, evaluated the economic impact of using PCV13 in lieu of PPV23 in all adults aged ≥50 y in the US. [55] A published model from the Netherlands estimated the cost-effectiveness of PCV13 compared to ‘no vaccination’ in adults’ aged ≥65 y. The results of the base case analysis indicated that PCV13 was cost-effective but not dominant. However, the model of Rozenbaum et al. had only a time horizon of five years. [56] We simulated a similar scenario in our model by reducing the revaccination frequency of PCV13 from 10 to 5 years. PCV13 would lose its dominance but still remained cost-effective compared to PPV23 as well as ‘no vaccination’.
Most of the previous mentioned health economic studies used the target population, vaccinated routinely in many countries. Effectiveness of PPV23 is discussed controversially. There is a homogeneous consensus that PPV23 has no protection against non-bacteremic pneumonia [9]. Only protection against IPD is assessed differently. Therefore we decided to us no decline in protection for both vaccines even if most of the PPV23 studies considered a decline in protection with different rates.
The focus of this study was to address the benefit of pneumococcal conjugate vaccination in adults. Adverse events are not a problem with conjugate vaccines. For PPV23 only a small impact of adverse events on ICER was shown. Therefore we decided not to implement cost for adverse events in the current model.
Considering the substantial morbidity, mortality and costs associated with pneumococcal disease as well as the limitations of Markov models to simulate the dynamics of transmissible disease, there is a need for more accurate models as soon as valid effectiveness data of PCV13 is published to confirm the cost-effectiveness of this vaccine.
Conclusions
In conclusion, our analysis indicates that adult PCV13 vaccination in Germany will reduce the burden of pneumococcal disease with substantial health and economic benefits when compared to the currently recommended PPV23 as well as ‘no vaccination’. However, final cost-effectiveness will depend mainly on efficacy data of PCV13 confirmed by clinical trials, particularly in inpatient CAP. Defining the risk population, vaccination rates, indirect (herd) effects and serotype replacement needs further research.
Appendix A
Sensitivity analysis
The tables below provide information on the parameters included in the probabilistic sensitivity analysis as well as additional results of the one-way and multi-way sensitivity analyses. The means, standard errors and the type of distributions of all parameters examined in the probabilistic analysis are shown in Additional file 1: Table S1. Additional file 2: Table S2 illustrates the results of all one-way and multi-way sensitivity analyses conducted.
References
Rueckinger S, van der Linden M, Reinert RR, von Kries R, Burckhardt F, Siedler A: Reduction in the incidence of invasive pneumococcal disease after general vaccination with 7-valent pneumococcal conjugate vaccine in Germany. Vaccine 2009, 27: 4136–4141. 10.1016/j.vaccine.2009.04.057
Conaty S, Watson L, Dinnes J, Waugh N: The effectiveness of pneumococcal polysaccharide vaccines in adults: a systematic review of observational studies and comparison with results from randomised controlled trials. Vaccine 2004, 22: 3214–3224. 10.1016/j.vaccine.2003.08.050
Cornu C, Yzebe D, Leophonte P, Gaillat J, Boissel JP, Cucherat M: Efficacy of pneumococcal polysaccharide vaccine in immunocompetent adults: a meta-analysis of randomized trials. Vaccine 2001, 19: 4780–4790. 10.1016/S0264-410X(01)00217-1
Fine MJ, Smith MA, Carson CA, Meffe F, Sankey SS, Weissfeld LA, Detsky AS, Kapoor WN: Efficacy of pneumococcal vaccination in adults. A meta-analysis of randomized controlled trials. Arch Intern Med 1994, 154: 2666–2677. 10.1001/archinte.1994.00420230051007
Go ES, Ballas ZK: Anti-pneumococcal antibody response in normal subjects: a meta-analysis. J Allergy Clin Immunol 1996, 98: 205–215. 10.1016/S0091-6749(96)70244-0
Huss A, Scott P, Stuck AE, Trotter C, Egger M: Efficacy of pneumococcal vaccination in adults: a meta-analysis. Canadian Medical Association Journal 2009, 180: 48–58. 10.1503/cmaj.080734
Hutchison BG, Oxman AD, Shannon HS, Lloyd S, Altmayer CA, Thomas K: Clinical effectiveness of pneumococcal vaccine. Meta-analysis. Can Fam Physician 1999, 45: 2381–2393.
Mangtani P, Cutts F, Hall AJ: Efficacy of polysaccharide pneumococcal vaccine in adults in more developed countries: the state of the evidence. Lancet Infect Dis 2003, 3: 71–78. 10.1016/S1473-3099(03)00514-0
Moberley S, Holden J, Tatham DP, Andrews RM: Vaccines for preventing pneumococcal infection in adults (Review). Cochrane Database Syst Rev 2008, CD000422. 10.1002/14651858.CD000422.pub2
Morre RA, Wiffen PJ, Lipsky BA: Are the pneumococcal polysaccharide vaccines effective? Meta-analysis of the prospective trials. BMC Publ Health 2000, 1: 1–10.
Puig-Barbera J, Belenguer VA, Goterris PM, Brines Benlliure MJ: Pneumococcal vaccine effectiveness in the elderly. Systematic review and meta-analysis. Aten Primaria 2002, 30: 269–281.
Watson L, Wilson BJ, Waugh N: Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults. Vaccine 2002, 20: 2166–2173. 10.1016/S0264-410X(02)00112-3
Paradiso PR, Gruber WC: The Potential of Pneumococcal Conjugate Vaccine in Adults. In Presented at the Sixth International Symposium on Pneumococci and Pneumococcal Diseases. Reykjavik, Iceland; 2011.
Statistisches Bundesamt (Hrsg.): Bevölkerung nach Altersgruppen, Familienstand und Religionszugehörigkeit. http://www.destatis.de
Robert Koch-Institut: Empfehlungen der Ständigen Impfkomission (STIKO). http://www.rki.de/cln_178/nn_2030884/DE/Content/Infekt/EpidBull/Archiv/2011/30__11,templateId=raw,property=publicationFile.pdf/30_11.pdf
Adam D, Busse A: Pneumokokkenkonjugatimpfstoffe: Expertenkonsensuspapier zum aktuellen Stand der Dinge. Monatsschrift Kinderheilkunde 2009, 157: 1252–1256. 10.1007/s00112-009-2132-5
de Roux A, Schmole-Thoma B, Siber GR, Hackell JG, Kuhnke A, Ahlers N, Baker SA, Razmpour A, Emini EA, Fernsten PD, Gruber WC, Lockhart S, Burkhardt O, Welte T, Lode HM: Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis 2008, 46: 1015–1023. 10.1086/529142
Schwarz TF, Flamaing J, Rumke HC, Penzes J, Juergens C, Wenz A, Jayawardene D, Giardina P, Emini EA, Gruber WC, Schmoele-Thoma B: A randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged >/=65 years. Vaccine 2011, 29: 5195–5202. 10.1016/j.vaccine.2011.05.031
Koch-Institut R: Mitteilung der Ständigen Impfkommission am Robert Koch-Institut Pneumokokken-Polysaccharid-Impfung – Anpassung der Empfehlung und Begründung. Epidemiologisches Bulletin 2009, 32: 337–338.
Statistisches Bundesamt: Sterbetafel 2007/9. http://www.destatis.de
Fleming DM, Elliot AJ: Estimating the risk population in relation to influenza vaccination policy. Vaccine 2006, 24: 4378–4385. 10.1016/j.vaccine.2006.02.053
Statistisches Bundesamt (Hrsg.): Diagnosedaten der Krankenhäuser nach Behandlungsort ab 2000 (Fälle/Sterbefälle, Pflegetage, durchschnittliche Verweildauer. Gliederungsmerkmale: Jahre, Behandlungsort, Alter, Geschlecht, Verweildauer, ICD10. http://www.gbe-bund.de
Institut für Qualität und Patientensicherheit: BQS-Qualitätsreport. 2008. http://www.bqs-qualitaetsreport.de/2008/ergebnisse/down
IMS Health Deutschland: Verschreibungsindex für Pharmazeutika (VIP). 2009. Unpublished Work
CapNetz: CAP Moratlitätsdaten. 2009. Unpublished Work
Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, Malinoski F, Madore D, Chang I, Kohberger R, Watson W, Austrian R, Edwards K: Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 2000, 19: 187–195. 10.1097/00006454-200003000-00003
Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, Noyes J, Lewis E, Ray P, Lee J, Hackell J: Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J 2002, 21: 810–815. 10.1097/00006454-200209000-00005
Hansen J, Black S, Shinefield H, Cherian T, Benson J, Fireman B, Lewis E, Ray P, Lee J: Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr Infect Dis J 2006, 25: 779–781. 10.1097/01.inf.0000232706.35674.2f
Maruyama T, Taguchi O, Niederman MS, Morser J, Kobayashi H, Kobayashi T, D’Alessandro-Gabazza C, Nakayama S, Nishikubo K, Noguchi T, Takei Y, Gabazza EC: Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. British Medical Journal 2010, 340: c1004. 10.1136/bmj.c1004
Reinert RR, Haupts S, van der Linden M, Heeg C, Cil MY, Al-Lahham A, Fedson DS: Invasive pneumococcal disease in adults in North-Rhine Westphalia, Germany, 2001–2003. Clin Microbiol Infect 2005, 11: 985–991. 10.1111/j.1469-0691.2005.01282.x
Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR: Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 2007, 369: 1179–1186. 10.1016/S0140-6736(07)60564-9
Metlay JP, Fishman NO, Joffe M, Edelstein PH: Impact of pediatric vaccination with pneumococcal conjugate vaccine on the risk of bacteremic pneumococcal pneumonia in adults. Vaccine 2006, 24: 468–475. 10.1016/j.vaccine.2005.07.095
Moore M, Pilishvili T, Farley MM: Trends in invasive pneumococcal pneumonia, selected U.S. Sites 1998–2006 [Abstract] n.A. 2008.
Ray GT, Whitney CG, Fireman BH, Ciuryla V, Black SB: Cost-effectiveness of pneumococcal conjugate vaccine: evidence from the first 5 years of use in the United States incorporating herd effects. Pediatr Infect Dis J 2006, 25: 494–501. 10.1097/01.inf.0000222403.42974.8b
Roche PW, Krause V, Cook H, Barralet J, Coleman D, Sweeny A, Fielding J, Giele C, Gilmour R, Holland R, Kampen R, Brown M, Gilbert L, Hogg G, Murphy D: Invasive pneumococcal disease in Australia, 2006. Commun Dis Intell 2008, 32: 18–30.
Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, Facklam RR, Jorgensen JH, Schuchat A: Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003, 348: 1737–1746. 10.1056/NEJMoa022823
Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, Smith PJ, Beall BW, Whitney CG, Moore MR: Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010, 201: 32–41. 10.1086/648593
Claes C, Reinert RR, von der Schulenburg JM: Cost effectiveness analysis of heptavalent pneumococcal conjugate vaccine in Germany considering herd immunity effects. Eur J Health Econ 2009, 10: 25–38. 10.1007/s10198-008-0098-1
World Health Organisation: Initiative for Vaccine Research (IVR). www.who.int
Institut für das Entgeltsystem im Krankenhaus: DRG Browser 2008_2010. 2010. http://www.g-drg.de
Imran MN, Leng PH, Yang S, Kurup A, Eng P: Early predictors of mortality in pneumococcal bacteraemia. Ann Acad Med Singapore 2005, 34: 426–431.
Kalin M, Ortqvist A, Almela M, Aufwerber E, Dwyer R, Henriques B, Jorup C, Julander I, Marrie TJ, Mufson MA, Riquelme R, Thalme A, Torres A, Woodhead MA: Prospective study of prognostic factors in community-acquired bacteremic pneumococcal disease in 5 countries. J Infect Dis 2000, 182: 840–847. 10.1086/315760
Kassenärztliche Bundesvereinigung: Einheitlicher Bewertungsmaßstab (EBM). http://www.kbv.de
Lauertaxe: Arzneimittelpreise. https://www.lauer-fischer.de/LF/Seiten/Verwaltung/Kundencenter.aspx
von der Schulenburg JM, Greiner W, Jost F, Klusen N, Kubin M, Leidl R, Mittendorf T, Rebscher H, Schoeffski O, Vauth C, Volmer T, Wahler S, Wasem J, Weber C: German Recommendations on Health Economic Evaluation: Third and Updated Version of the Hanover Consensus. Value Health 2008, 11: 539–544. 10.1111/j.1524-4733.2007.00301.x
Statistisches Bundesamt: Arbeitsunfähigkeit bei AOK-Pflichtmitgliedern ohne Rentner (Arbeitsunfähigkeitsfälle, Arbeitsunfähigkeitstage, Tage je Fall). Gliederungsmerkmale: Jahre, Deutschland, Geschlecht, ICD-10. http://www.destatis.de
Statistisches Bundesamt (Hrsg.): Bevölkerung, Erwerbstätige, Erwerbslose, Erwerbspersonen, Nichterwerbspersonen: Deutschland, Jahre, Altersgruppen. http://www.destatis.de
Schnoor M, Klante T, Beckmann M, Robra BP, Welte T, Raspe H, Schafer T: Risk factors for community-acquired pneumonia in German adults: the impact of children in the household. Epidemiol Infect 2007, 135: 1389–1397.
Jackson L, Rice K, Lauksens K, Greenberg R, Jones T, Jayawardene D, Love J, Scott D, Emini E, Gruber W, Schoele-Thoma B: Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Presented at the 21st European Congress of Clinical Microbiology and Infectious Diseases (ECCMID)/27th International Congress ofChemotherapy (ICC), Milan, Italy; 2011. oral presentation
Jackson L, Gurtman A, van Cleeff M, Jansen K, Jayawardene D, Devlin C, Scortt D, Emini E, Gruber W, Schmoele-Thoma B: Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in pneumococcal naïve adults 50 through 64 years of age. Presented at the 21st European Congress of Clinical Microbiology and Infectious Diseases (ECCMID)/27th International Congress of Chemotherapy (ICC), Milan, Italy; 2011. oral presentation
van der Linden M, Imoehl M: Invasive Pneumokokkenerkrankungen bei Erwachsenen in Deutschland: Bestandsaufnahme vier Jahre nach Einführung der Pneumokokken-Konjugatimpfung bei Kindern [Abstract] Presented at the 2nd National Vaccination Conference in Stuttgart vom. 2011.
Ardanuy C, Tubau F, Pallares R, Catalayud L, Dominguez MA, Rolo D, Grau I, Martín R, Linares J: Epidemiology of invasive pneumococcal disease among adult patients in Barcelona before and after pediatric 7-valent pneumococcal conjugate vaccine introduction, 1997–2007. Clin Infect Dis 2011, 48: 57–64.
van der Linden M: Replacement of serotypes in Germany. Personal Communication, ;
Ogilvie I, Khoury AE, Cui Y, Dasbach E, Grabenstein JD, Goetghebeur M: Cost-effectiveness of pneumococcal polysaccharide vaccination in adults: a systematic review of conclusions and assumptions. Vaccine 2009, 27: 4891–4904. 10.1016/j.vaccine.2009.05.061
Weycker D, Sato R, Strutton D, Edelsberg J, Jackson LA: Public Health and Economic Impact of PCV13 in US Adults Aged > = 50. Infectious Diseases Society of America 2010., 462:
Rozenbaum MH, Hak E, van der Werf TS, Postma MJ: Results of a cohort model analysis of the cost-effectiveness of routine immunization with 13-valent pneumococcal conjugate vaccine of those aged > or =65 years in the Netherlands. Clin Ther 2010, 32: 1517–1532. 10.1016/j.clinthera.2010.06.016
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The paper was supported by Pfizer Deutschland GmbH. Mathias Pletz is a member of the Pfizer advisory board and has received research grants of Sanofi Pasteur MSD.
Authors’ contributions
AK constructed and implemented the model in Excel, performed the analysis of the results and drafted the manuscript. UT was responsible for the literature review, data acquisition and drafted the manuscript. MP helped to draft the manuscript and revised it critically for important intellectual content. JMGvdS reviewed the manuscript and revised it critically for important intellectual content. All authors read and approved the final manuscript.
Electronic supplementary material
13561_2011_32_MOESM1_ESM.doc
Additional file 1: Table S1. Means, standard errors and distributions of parameters examined in the probabilistic sensitivity analysis. (DOC 140 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kuhlmann, A., Theidel, U., Pletz, M.W. et al. Potential cost-effectiveness and benefit-cost ratios of adult pneumococcal vaccination in Germany. Health Econ Rev 2, 4 (2012). https://doi.org/10.1186/2191-1991-2-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2191-1991-2-4