Abstract
Metaplastic breast carcinoma (MBC) is an uncommon malignancy characterized by the co-existence of two or more cellular types, commonly a mixture of epithelial and mesenchymal components. A case of a female patient aged 46 years with MBC (carcinosarcoma) is presented, including mammographic, ultrasonic, gross examination, and pathological findings. After undergoing modified radical mastectomy of the left breast and subsequent six courses of adjuvant chemotherapy and endocrine therapy, the patient is now doing well with no recurrence and metastasis. Conventional treatments for invasive ductal carcinoma (IDC) may appear to be less effective. Patients with MBC would be appropriate candidates for innovative or targeted therapy regimens.
Similar content being viewed by others
Background
Metaplastic breast carcinoma (MBC) is a rare and heterogeneous group of malignancies that constitutes less than 1% of all breast cancers [1–3]. The World Health Organization recognized and classified metaplastic carcinoma in 2003 [4]. We report our experience with a case of a 46-year-old female who had a mixed epithelial and mesenchymal metaplastic carcinoma (carcinosarcoma) of the left breast, and we also present here a short review of the literature.
Case presentation
A 46-year-old woman with two lumps in the upper outer quadrant of the left breast was referred to our department. The patient complained that the painless lumps have been growing rapidly over the previous three weeks. She had no history of trauma, nipple discharge, or other previous breast diseases. There was no known family history of breast cancer.
On clinical examination, palpation revealed two firm and mobile lumps closely adjacent to each other, measuring about 4.0 × 4.0 cm and 3.0 × 3.0 cm, respectively. There was no dimpling or puckering of the skin or changes of the skin color and the nipple. Axillary lymph nodes and other superficial lymph nodes were not palpable. Contralateral breast and axilla were normal.
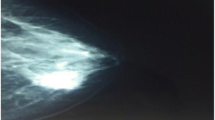
Mammography revealed two well-circumscribed, round masses in the upper outer quadrant of the left breast, measuring 4.7 × 4.5 cm and 4.2 × 3.8 cm, respectively. The masses were generally high density, accompanied by heterogeneous micro-calcifications. The lesion corresponded to category 4B according to the BI-RADS Mammography lexicon classification [5] (Figure 1). Ultrasound demonstrated a pear-shaped, complex echoic lesion measuring approximately 8.3 × 3.7 × 7.3 cm with relatively indistinct margins in the upper outer quadrant of the left breast. One hypoechoic mass at the 2 o’clock position of the lesion was accompanied by a hyperechoic area with abundant vessels within the mass. The 3 o’clock mass of the lesion was hyperechoic with spotted blood flow. No enlarged lymph nodes were detected. An ultrasound diagnosis of intraductal papilloma accompanied by hemorrhage and solid mass (BI-RADS 4) [5] was made (Figure 2). The whole lesion was blue colored, with an elasticity score of 4 (Figure 3). Though no lymph nodes were detected in either clinical or ultrasonic examination, the patient did not agree with substituting axillary lymph node dissection (ALND) by sentinel lymph node biopsy (SLNB). Thus, modified radical mastectomy with ALND was performed. Gross examination of the specimen revealed a cystic-solid tumor with complete envelope, which consisted of two parts. The tumor was measured 7.0 cm in length and width, and 5.0 cm in height with the solid part measured 4.0 × 4.0 × 3.5 cm. Dark-red intracystic hemorrhage was noted (Figure 4). Microscopically, the tumor exhibited a biphasic pattern consisting of epithelial and mesenchymal components. Intraductal cell masses were formed in the epithelial components with obvious heteromorphism and central necrosis. Meanwhile, a large number of spindle cells with some multinucleate giant cells were present in the mesenchymal components in an interlaced pattern (Figure 5). Pathological diagnosis was mixed epithelial and mesenchymal metaplastic carcinoma (carcinosarcoma, histological grade III). No metastasis was found in 15 lymph nodes.
Immunohistochemically (images not included), the breast tumor showed negative for Cytokeratin (CK, CK5/6, and CK7), Desmin, S-100, and CD34 but showed positive for Vimentin, SMA, and CD68. However, the epithelial components expressed positive estrogen receptor (ER), progesterone receptor (PR), and C-erbB-2. In contrast, estrogen and progesterone receptors were negative, with weakly positive C-erbB-2, in the mesenchymal components. Ki-67 expression was 20%.
Having undergone three cycles of CEF (cyclophosphamide, epirubicin, and fluorouracil) and three cycles of T (Taxotere) followed by Tamoxifen, the patient is doing well one year after surgery with no recurrence and metastasis.
Discussion
Accounting for less than 1% of breast cancers diagnosed annually [1–3], metaplastic breast carcinoma (MBC) is known to be characterized by the presence of two or more cellular elements histologically, commonly a mixture of epithelial and mesenchymal components [6–10]. Wargotz and Norris suggested that carcinosarcoma of the breast, in which an epithelial-mesenchymal transition zone does not exist, should be distinguished from other MBC diseases. Such diagnosis is not difficult with detailed histological investigation [7]. Classification of metaplastic carcinoma was proposed by the World Health Organization in 2003 as 1) squamous cell carcinoma, 2) adenocarcinoma with spindle cell proliferation, 3) adenosquamous, including mucoepidermoid, and 4) mixed epithelial and mesenchymal. Subtypes of mixed epithelial and mesenchymal carcinoma includes a) carcinoma with chondroid metaplasia, b) carcinoma with osseous metaplasia, and c) carcinosarcoma [4]. Carcinosarcoma is a general term describing biphasic lesions that simultaneously contain malignant epithelial and malignant mesenchymal tissue components [11]. The origin of breast carcinosarcoma is far from clear. They have been reported to develop from existing cystosarcoma phyllodes, fibroadenoma and cystic backgrounds [12–14]. Carcinosarcoma is characterized by the loss of intercellular adhesion, down-regulation of epithelial makers (cytokeratins), upregulation of mesenchymal markers [vimentin and smooth muscle actin (SMA)], increase in motility, invasiveness, and metastatic capabilities [15–19].
Although relatively rare and histologically heterogeneous, their clinical manifestations are often similar. They are often palpable, mobile, and large, showing benign imaging features such as a round or oval shape with circumscribed margins. Meanwhile, estrogen, progesterone, and Her-2 are less frequently shown to be positive, with lower rates of axillary node involvement [20–24]. But approximately 70% of metaplastic carcinomas show epidermal growth factor receptor (EGFR) gene amplification and overexpression [25].
The optimal treatment strategies for MBC are unknown. Currently, management of MBC has largely paralleled that of invasive ductal carcinoma (IDC). Theoretically, MBC patients should undergo mastectomy rather than lumpectomy due to larger masses compared to their IDC counterparts. However, studies have found no difference in overall or disease-free survival between patients with MBC undergoing either modified radical mastectomy or breast conservation therapy [26, 27]. Traditional adjuvant chemotherapy for IDC is ineffective against MBC [28–30], so is hormonal therapy as there is a high incidence of hormone receptor negativity in MBC [31]. Tseng et al. suggested that adjuvant radiation improved both overall and disease-specific survival for all patients undergoing treatment for MBC, regardless of the type of operation performed (lumpectomy versus mastectomy) [26]. Treatment given in the neoadjuvant setting has become the standard approach for potentially operable breast carcinomas with benefits including tumor downsizing, earlier treatment of micrometastatic disease, and the ability to assess responsiveness to therapy directly. However, it is important to identify patients who would benefit from this approach and those who would not. We should be cautious when considering neoadjuvant chemotherapy given to those with MBC because several studies indicated that these patients responded poorly and might gain less benefit from standard regimens [20, 32, 33]. In addition, samples obtained by either fine needle aspiration or core needle biopsy might not be sufficient to distinguish MBC from common types of breast cancer, which suggests that it could be difficult to make an accurate diagnosis preoperatively [21, 34, 35]. Therefore, conventional neoadjuvant regimens, if given to patients with MBC, may carry the risks of tumor progression. Takuwa et al. reported a case of MBC that responded well to platinum-based neoadjuvant chemotherapy, resulting in nearly a pathologically complete response [36]. Nevertheless, careful monitoring is essential since the failure of chemotherapy may result in clinical deterioration and preoperative complications. Under the circumstance of ineffectiveness of conventional therapies, innovative or targeted treatments are being explored. Since MBC tends to be EGFR positive while Her-2 tends to be negative, Leibl and Moinfar suggested that targeted protein kinase inhibitors such as gefitinib might be effective [25].
The prognosis of MBC still remains controversial. Some studies reported that compared to patients with IDC, those with MBC have a worse prognosis. Their overall survival and disease-free survival are both decreased despite presenting more commonly as node-negative disease [37, 38]. Conversely, other studies showed comparable outcomes with matched typical breast cancer [2, 21]; however, almost all MBC recurrences happened during the first five years, whereas recurrence curves for IDC continued to fall over time, suggesting the possibility that MBC may show an earlier recurrence than IDC [21].
Conclusions
Generally, MBC is often palpable, mobile, and large, showing a round or oval-shaped mass with a circumscribed margin in the mammogram. Given the heterogeneity of MBC, an accurate preoperative diagnosis may not be achieved with needle biopsies. Conventional treatments for IDC also appear to be less effective. Patients with MBC would be appropriate candidates for innovative or targeted therapy regimens. Prospective studies are needed, but the rarity of MBC makes these less likely to be conducted.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Abbreviations
- ALND:
-
axillary lymph node dissection
- HE:
-
hematoxylin and eosin stain
- ER:
-
estrogen receptor
- IDC:
-
invasive ductal carcinoma
- MBC:
-
metaplastic breast carcinoma
- PR:
-
progesterone receptor
- SLNB:
-
sentinel lymph node biopsy.
References
Oberman HA: Metaplastic carcinoma of the breast. A clinicopathologic study of 29 patients. Am J Surg Pathol 1987, 11: 918–929. 10.1097/00000478-198712000-00002
Chao TC, Wang CS, Chen SC, Chen MF: Metaplastic carcinomas of the breast. J Surg Oncol 1999, 71: 220–225. 10.1002/(SICI)1096-9098(199908)71:4<220::AID-JSO3>3.0.CO;2-L
Park JH, Han W, Kim SW, Lee JE, Shin HJ, Kim SW, Choe KJ, Oh SK, Youn YK, Noh DY: The clinicopahologic characteristics of 38 metaplastic carcinomas of the breast. J Breast Cancer 2005, 8: 59–63.
Tavassoli FA, Devilee P World Health Organization Classification of Tumours. In Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Volume 4. 3rd edition. Lyon, France: IARC Press; 2003.
American College of Radiology: Breast imaging reporting and data system. 4th edition. Reston, VA: American College of Radiology; 2003.
Wargotz ES, Deos PH, Norris HJ: Metaplastic carcinomas of the breast. II. Spindle cell carcinoma. Hum Pathol 1989, 20: 732–740. 10.1016/0046-8177(89)90065-8
Wargotz ES, Norris HJ: Metaplastic carcinomas of the breast. III. Carcionsarcoma. Cancer 1989, 64: 1490–1499. 10.1002/1097-0142(19891001)64:7<1490::AID-CNCR2820640722>3.0.CO;2-L
Wargotz ES, Norris HJ: Metaplastic carcinomas of the breast. I. Matrix-producing carcinoma. Hum Pathol 1989, 20: 628–635. 10.1016/0046-8177(89)90149-4
Wargotz ES, Norris HJ: Metaplastic carcinomas of the breast. V. Metaplastic carcinomas with osteoclastic giant cells. Hum Pathol 1990, 21: 1142–1150. 10.1016/0046-8177(90)90151-T
Wargotz ES, Norris HJ: Metaplastic carcinomas of the breast. IV. Squamous cell carcinoma of ductal orgin. Cancer 1990, 65: 272–276. 10.1002/1097-0142(19900115)65:2<272::AID-CNCR2820650215>3.0.CO;2-6
Millis RR, Hanby AM, Girling AC: Diagonstic surgical pathology. 2nd edition. New York: Raven Press; 1994:374–376.
Harris M, Persaud V: Carcionsarcoma of the breast. J Pathol 1974, 112: 99–105. 10.1002/path.1711120205
Bolton B, Sieunarine K: Carcinosarcoma: a rare tumour of the breast. Aust N Z J Surg 1990, 60: 917–919. 10.1111/j.1445-2197.1990.tb07501.x
Teixeira MR, Ovist H, Bohler PJ, Pandis N, Heim S: Cytogenetic analysis shows that carcinosarcomas of the breast are of monoclonal origin. Genes Chromosomes Cancer 1998, 22: 145–151. 10.1002/(SICI)1098-2264(199806)22:2<145::AID-GCC9>3.0.CO;2-X
Thiery JP: Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002, 2: 442–454. 10.1038/nrc822
Thompson EW, Newgreen DF, Tarin D: Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res 2005, 65: 5991–5995.
Thiery JP, Sleeman JP: Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006, 7: 131–142. 10.1038/nrm1835
Gupta GP, Massague J: Cancer metastasis: building a framework. Cell 2006, 127: 679–695. 10.1016/j.cell.2006.11.001
Savagner P: Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal trasition. Bioessays 2001, 23: 912–923. 10.1002/bies.1132
Tamura N, Kinoshita T: A cast of metaplastic carcinoma of the breast. Jpn J Clin Oncol 2011, 41: 1045. 10.1093/jjco/hyr107
Park HS, Park S, Kim JH, Lee JH, Choi SY, Park BW, Lee KS: Clinicopathologic features and outcomes of metaplastic breast carcinoma: comparison with invasive ductal carcinoma of the breast. Yonsei Med J 2010, 51: 864–869. 10.3349/ymj.2010.51.6.864
Smitt MC: Metaplastic breast cancer. Clin Breast Cancer 2003, 4: 210–211.
Li S, Wei QZ: Metaplastic carcinoma of the right breast and simultaneous giant ovarian teratoma: a case report. Chin J Cancer 2012, 31: 500–504. 10.5732/cjc.012.10118
Yang WT, Hennessy B, Broglio K, Mills C, Sneige N, Davis WG, Valero V, Hunt KK, Gilcrease MZ: Imaging differences in metaplastic and invasive ductal carcinomas of the breast. AJR Am J Roentgenol 2007, 189: 1288–1293. 10.2214/AJR.07.2056
Leibl S, Moinfar F: Metaplastic breast carcinomas are negative for Her-2 but frequently express EGFR (Her-1): potential relevance to adjuvant treatment with EGFR tyrosine kinase inhibitors? J Clin Pathol 2005, 58: 700–704. 10.1136/jcp.2004.025163
Tseng WH, Martinez SR: Metaplastic breast cancer: to radiate or not to radiate? Ann Surg Oncol 2001, 18: 94–103.
Dave G, Cosmatos H, Do T, Lodin K, Varshney D: Metaplastic carcinoma of the breast: a retrospective review. Int J Radiat Oncol Biol Phys 2006, 64: 771–775. 10.1016/j.ijrobp.2005.08.024
Pezzi CM, Patel-Parekn L, Cole K, Franko J, Klimberg VS, Bland K: Characteristics and treatment of metaplastic breast cancer: analysis of 8892 cases from the national cancer data base. Ann of Surg Oncol 2007, 14: 166–173.
Rayson D, Adjei AA, Suman VJ, Wold LE, Ingle JN: Metaplastic breast cancer: prognosis and response to systemic therapy. Ann Oncol 1999, 10: 413–419. 10.1023/A:1008329910362
Hennessy BT, Giordano S, Broglio K, Duan Z, Trent J, Buchholz T, Babiera G, Hortobagyi GN, Valero V: Biphasic metaplastic sarcomatoid carcinoma of the breast. Ann Oncol 2006, 17: 605–613. 10.1093/annonc/mdl006
Bae SY, Lee SK, Koo MY, Hur SM, Choi MY, Cho DH, Kim S, Choe JH, Lee JE, Kim JH, Kim JS, Nam SJ, Yang JH: The prognoses of metaplastic breast cancer patients compared to those of triple-negative breast cancer patients. Breast Cancer Res Treat 2011, 126: 471–478. 10.1007/s10549-011-1359-8
Nagao T, Kinoshita T, Hojo T, Tsuda H, Tamura K, Fujiwara Y: The differences in the histological types of breast cancer and the response to neoadjuvant chemotherapy: the relationship between the outcome and the clinicopathological characteristics. Breast 2012, 21: 289–295. 10.1016/j.breast.2011.12.011
Chen IC, Lin C, Huang CS, Lien HC, Hsu C, Kuo WH, Lu YS, Cheng AL: Lack of efficacy to systemic chemotherapy for treatment of metaplastic carcinoma of the breast in the modern era. Breast Cancer Res Treat 2011, 130: 345–351. 10.1007/s10549-011-1686-9
Lale S, Kure K, Lingamfelter D: Challenges to diagnose metaplastic carcinoma of the breast through cytologic metods: an eight-case series. Diagn Pathol 2011, 6: 7. 10.1186/1746-1596-6-7
Beatty JD, Atwood M, Tickman R, Reiner M: Metaplastic breast cancer: clinical significance. Am J Surg 2006, 191: 657–664. 10.1016/j.amjsurg.2006.01.038
Takuwa H, Ueno T, Ishiguro H, Mikami Y, Kanao S, Takada M, Sugie T, Toi M: A case of metaplastic breast cancer that showed a good response to platinum based preoperative chemotherapy. Breast Cancer 2011,. PMID: 21526425
Luini A, Aguilar M, Gatti G, Fasani R, Botteri E, Brito JA, Maisonneuve P, Vento AR, Viale G: Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: the experience of the European Institute of Oncology and review of the literature. Breast Cancer Res Treat 2007, 101: 349–353. 10.1007/s10549-006-9301-1
Esses KM, Hagmaier RM, Blanchard SA, Lazarchick JJ, Riker AI: Carcinosarcoma of the breast: two case reports and review of the literature. Cases J 2009, 2: 15. 10.1186/1757-1626-2-15
Acknowledgements
This work was partially supported by grants from the National Natural Science Foundation of China (No. 81071900, 81172199 and 81272920).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YK drafted the manuscript and revised it. SK, QCL, and XYZ were responsible for images and corresponding interpretations. XYZ contributed to manuscript proofreading and revisions. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kang, Y., Kang, S., Li, Q. et al. Mixed epithelial and mesenchymal metaplastic carcinoma (carcinosarcoma) of the breast: a case report. Eur J Med Res 19, 14 (2014). https://doi.org/10.1186/2047-783X-19-14
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2047-783X-19-14