Abstract
Introduction
Peripheral giant cell granuloma and peripheral ossifying fibroma are clinicopathologically distinct gingival lesions. Both are included in clinical differential diagnoses of common benign and reactive gingival epulides in humans. It is often impossible to make a clinical distinction between the two entities, thereby making definitive diagnosis dependent on histopathologic features. While our search of the English literature revealed several reports of peripheral giant cell granuloma with ‘bone formation’, we were unable to identify any reports of hybrid peripheral ossifying fibroma-peripheral giant cell granulomas.
Case presentation
We report a case of a 44-year-old Caucasian man presenting with a three-month history of swelling of his right posterior mandible, related to an area of previous dental implant restoration. A clinical examination revealed modest extraoral facial swelling of his right posterior mandible, while an intraoral examination showed a 45×25×15mm sessile, lobular soft tissue mass of the right posterior mandibular gingiva. The mucosal covering of the lesion exhibited focal surface ulceration. A panoramic radiograph showed two implants at the vicinity of the lesion with no other significant findings. An excisional biopsy of the lesion followed by histopathologic examination of the biopsy specimen revealed salient and distinctive features of peripheral giant cell granuloma and of peripheral ossifying fibroma, estimated at near equal proportions. This raises the possibility of a hybrid odontogenic lesion.
Conclusion
The presentation of this lesion, with areas of peripheral giant cell granuloma along with a distinct area of extensive osseous formation and stroma reminiscent of a peripheral ossifying fibroma, justifies consideration of this as a possible hybrid lesion. Although the biologic behavior of a combined lesion is not anticipated to deviate significantly from that of either of the single entities, this case resurrects an enduring debate as to whether peripheral giant cell granuloma and peripheral ossifying fibroma are simply parts of a disease spectrum, or whether some of these lesions represent true hybrid lesions. It is therefore recommended that more cases with histopathologic features similar to the lesion in our case be reported in the literature to further elucidate the histogenesis of these lesions.
Similar content being viewed by others
Introduction
Peripheral ossifying fibroma (POsF) was first reported and described by Shepherd as ‘alveolar exostosis’ in 1844 [1]. Because this lesion presents with a spectrum of histomorphologic features, several subsequent reports have characterized it variously as peripheral fibroma with calcification, calcifying fibroblastic granuloma, ossifying fibroid epulis, peripheral cemento-ossifying fibroma, and calcifying fibroma [2, 3]. The terminology has since stabilized, with this entity now referred to as POsF, peripheral cementifying fibroma, or peripheral cement-ossifying fibroma, depending on whether bone, cementum, or proportions of each are present on microscopy [3–7]. Similarly, peripheral giant cell granuloma (PGCG) was first reported as fungus flesh in 1848 [4], then reported as giant cell reparative granuloma by Jaffe in 1953 [8]. Subsequent reports also featured a constellation of terminology such as osteoclastoma, giant cell epulis, and myeloid epulis [3]. PGCG is now the preferred terminology.
POsF and PGCG are site-specific lesions arising exclusively from the periodontal ligament. This makes both, by definition, lesions of the gingiva or alveolar ridge [9, 10]. Their histogenesis is distinct from that of their respective intraosseous (central) namesakes, central ossifying fibroma (COF) and central giant cell granulomas (CGCG), which are intra-bony benign neoplasms of the jawbone [10]. Thus, POsF and PGCG are regarded as reactive lesions of gingiva, often presenting as painless, lobular, and ulcerated masses that are clinically indistinguishable from one another.
Cases of both POsF and of PGCG are occasionally seen with isolated foci of the diagnostic histopathologic feature of the other, but the preferred diagnosis of one over the other is usually based on the predominant morphologic features present in any specific case. Thus, cases with predominantly stromal cells with numerous osteoclast-like multinucleated giant cells with only focal areas of calcified bone and/or cementum deposits are diagnosed as PGCG. Those with a stroma comprising aggregates of primitive oval and bipolar mesenchymal cells, where a trabecular of woven and lamella bone with cementum-like deposit often dominates, receive a POsF diagnosis [11]. Archival papers by Dayan et al. [12] and similar reports by Katsikeris et al. [13] highlighted the overlaps in the histopathologic features of PGCG and POsF. Here, we report on a case of PGCG with a distinct region with an extensive osseous component and other stromal features of POsF that may qualify this lesion as a true case of hybrid PGCG-POsF. We also discuss the pathogenesis of this lesion.
Case presentation
A 44-year-old Caucasian man presented to our Urgent Care clinic with a complaint of swelling in his right posterior mandibular molar teeth area that started about three months previously. There was no associated pain except for occasional interference of the swelling with occlusion and mastication. His vital signs were within normal ranges; his medical history included a family history of diabetes, cardiovascular disease, and cancers. His previous dental treatment included two right posterior quadrant dental implants, proximate to the lesion area, placed three months prior to presentation.
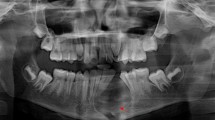
On clinical examination, he had slight extraoral facial swelling of his right posterior mandible, but his regional lymph nodes were not palpable. An intraoral examination showed a 45×25×15mm sessile, lobular soft tissue mass of his right posterior mandibular gingiva related to his first and second premolars (Figure 1). The mucosal covering of the lesion exhibited surface tan, red, and bluish areas with a focal area of ulceration. A panoramic radiograph of his jaws revealed two implants at his right posterior mandible in the vicinity of the lesion (Figure 2) with no other significant findings. These clinical and radiographic findings indicated a benign lesion, and the following differential diagnoses were generated: pyogenic granuloma, POsF, peripheral odontogenic fibroma, focal fibroepithelial hyperplasia, and PGCG. Malignant entities such as squamous cell carcinoma, other primary malignant lesions, and metastatic lesions, although thought to be unlikely, were also considered. Complete excision of the lesion was performed, and the entire specimen submitted for histopathologic examination.
Microscopic examination of the hematoxylin and eosin-stained sections of the specimen revealed a nodular soft tissue specimen consisting of an ulcerated benign cellular mesenchymal tissue proliferation supporting elaborate trabecular bone formation, and occasional cementum-like calcified deposits (Figure 3A,B,D). This interfaced with stroma supporting aggregates of benign multinucleated giant cells with hemorrhagic and hemosiderin deposits (Figure 3A-3C). This framework was covered by discontinuous parakeratinized surface stratified squamous epithelium maintaining the usual pattern of cellular maturation, which rested on an intact basal cell layer with elongated rete ridges. Where the surface epithelium was discontinuous it was covered by a fibrinous layer supporting neutrophils and extravasated erythrocytes.
Histopathologic micrograph of combined peripheral ossifying fibroma and peripheral giant cell granuloma. Hematoxylin and eosin-stained sections showing PGCG and POsF areas of lesion at (A) 10× magnification and (B) 20× magnification. (C) Same staining predominantly showing the PGCG area and prominent aggregates of multinucleated giant cell reaction (*) at 40× magnification. (D) Same staining showing a predominantly POsF area (arrow) within the hybrid lesion at 40× magnification. POsF, peripheral ossifying fibroma; PGCG, peripheral giant cell granuloma.
Discussion
Various combinations of so-called hybrid odontogenic lesions have been reported [14]. These include reports of several series on combined COF with CGCG [14–16]. For example, Allen et al. [15] reported three cases of ‘epithelium-rich’ COF, with ‘unusual associated giant cell reaction’, whereas Tosios et al. [14] reported seven cases of hybrid central giant cell lesions (CGCLs) and COFs of the jaws. Odell et al. [16] gave a detailed microscopic description of eight patients with ‘hybrid central giant cell granuloma and central odontogenic fibroma-like’ lesions. There are also reports of CGCL with ameloblastoma, and several non-odontogenic fibro-osseous lesions [17, 18]. Although the case series reported by Dayan et al. [12] and Katsikeris et al. [13] highlighted the formation of mineralized tissues in PGCG, they did not find cementum-like calcifications in the mineralized matrix in their respective series. To the best of our knowledge, our case is the first reported in which the PGCG area is distinct from the mineralized area and present in near equal proportion, and the mineralized stroma contains occasional cementum-like matrix.
POsFs are reactive lesions distinct from COFs, which are true intraosseous neoplasms. Similarly, PGCGs are reactive lesions that do not represent the intraosseous counterpart of CGCG. In both pairings the peripheral lesions present as indistinguishable histopathologic look-alikes, as do the corresponding central lesions. Because of the contiguity of the gingival and alveolar apparatus to the alveolar bone, mandible, and maxilla, the presentation of these entities occasionally raises the possibility of either an intra-bony (mandible or maxilla) lesion that eroded to the surface and into the overlying gingival soft tissue, or an originally soft tissue lesion that burrowed its way into bone [11]. In our case there was no radiographic evidence of alveolar bone involvement, thereby establishing the diagnosis of a soft tissue entity.
Dayan et al. [12] emphasized the absence of cementum deposits or matrix in PGCG with extensive osseous elements as a basis for characterizing these lesions as PGCG with osseous formation or metaplasia, instead of as true hybrid PGCG-POsF lesions. While we agree that cases of osseous metaplasia in PGCG may be common, we suggest that the absence of cementum-like elements alone is not sufficient to reject all such lesions as true PGCG-POsF hybrid lesions. This is because cases of POsF lacking distinct cementum-like deposits are not uncommon. Thus a diagnosis of POsF is based on the presence of distinct and characteristic stroma, comprising intertwining bundles of collagen admixed with haphazardly arranged fibroblast-like cells, in association with bone and/or cementum-like material. Our case showed a distinct area satisfying the histopathologic features of POsF (Figure 3D). The POsF area was unlikely to represent ‘exuberant callus’ because, although common in long bones, this is almost never seen at the surface of jaw bones [11]. Furthermore, there was a noticeable paucity of red cell extravasation in the stroma of the POsF portion of the lesion compared with the PGCG portion (Figure 3C), which showed characteristic exuberance of red cell extravasation accompanied by hemosiderin deposits [11].
Both POsF and PGCG are reactive inflammatory hyperplasias arising from the pluripotent cells of the periodontal ligaments [11]. It is therefore conceivable that both entities represent points on a spectrum of the same reactive disease process. While the implant treatment in the vicinity of the lesion may have constituted a persistent inflammatory trigger or irritant, we suggest the possibility that the POsF and PGCG arose de novo. Cases of PGCG associated with dental implant therapies have been reported [19–21]. In most of the cases the implant and restoration materials were composed of coronal prosthesis and were associated with poor oral hygiene [20, 21].
Conclusion
We report a case of PGCG with a distinct area of extensive osseous formation and stromal area, reminiscent of that characteristic of POsF. The combined classic histopathologic features of both lesions represented in similar proportions justify the diagnosis of a combined PGCG-POsF. The biologic behavior of the combined lesion is not anticipated to deviate significantly from that of either of the lesions in their commonly single entity presentations. We recommend that more cases with histopathologic features similar to our case be reported in the literature to further elucidate the histogenesis of these lesions.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Abbreviations
- CGCL:
-
central giant cell lesion
- COF:
-
central ossifying fibroma
- PGCG:
-
peripheral giant cell granuloma
- POsF:
-
peripheral ossifying fibroma.
References
Shepherd SM: Alveolar exostosis.Am J Dent Sci 1884, 4:43–44.
Bhaskar SN, Jacoway JR: Peripheral fibroma and peripheral fibroma with calcification: report of 376 cases.J Am Dent Assoc 1966, 73:1312–1320. 10.14219/jada.archive.1966.0375
Buchner A, Hansen LS: The histomorphologic spectrum of peripheral ossifying fibroma.Oral Surg Oral Med Oral Pathol 1987, 63:452–461. 10.1016/0030-4220(87)90258-1
Tomes J: A course of lectures on dental physiology and surgery (lectures I-XV).Am J Dent Sci 1846–1848, 7:1–68. 121–134, 33–54, 120–147, 313–350
Zain RB, Fei YJ: Fibrous lesion of the gingiva: a histopathologic analysis of 204 cases.Oral Surg Oral Med Oral Pathol 1990, 70:466–470. 10.1016/0030-4220(90)90212-B
Feller L, Buskin A, Raubenheimer EJ: Cemento-ossifying fibroma: case report and review of the literature.J Int Acad Periodontol 2004, 6:131–135.
Kumar SK, Ram S, Jorgensen MG, Shuler CF, Sedghizadeh PP: Multicentric peripheral ossifying fibroma.J Oral Sci 2006, 48:239–243. 10.2334/josnusd.48.239
Jaffe HL: Giant-cell reparative granuloma, traumatic bone cyst, and fibrous (fibro-osseous) dysplasia of the jawbones.Oral Surg Oral Med Oral Pathol 1953, 6:159–175. 10.1016/0030-4220(53)90151-0
Cawson RA, Speight P, Binnie WH, Wright J: Lucas’s Pathology of the Oral Tissues. 5th edition. Philadelphia: WB Saunders; 1998.
Neville BW, Aamm DD, Allen CM, Bouquot JE: Oral and Maxillofacial Pathology. 3rd edition. Philadelphia: WB Saunders; 2008.
Bouquot JE, Muller S, Nikai H: Lesions of the oral cavity. In Diagnostic Surgical Pathology of the Head and Neck. 2nd edition. Edited by: Gnepp DR. Philadelphia: WB Saunders; 2009:191–308.
Dayan D, Buchner A, Spirer S: Bone formation in peripheral giant cell granuloma.J Periodontol 1990, 61:444–446. 10.1902/jop.1990.61.7.444
Katsikeris N, Kakarantza-Angelopoulou E, Angelopoulos AP: Peripheral giant cell granuloma: clinicopathologic study of 224 new cases and review of 956 reported cases.Int J Oral Maxillofac Surg 1988, 17:94–99. 10.1016/S0901-5027(88)80158-9
Tosios KI, Gopalakrishnan R, Koutlas IG: So-called hybrid central odontogenic fibroma/central giant cell lesion of the jaws. A report on seven additional cases, including an example in a patient with cherubism, and hypotheses on the pathogenesis.Head Neck Pathol 2008, 2:333–338. 10.1007/s12105-008-0076-z
Allen CM, Hammond HL, Stimson PG: Central odontogenic fibroma, WHO type: a report of three cases with an unusual associated giant cell reaction.Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1992, 73:62–66. 10.1016/0030-4220(92)90156-K
Odell EW, Lombardi T, Barrett AW, Morgan PR, Speight PM: Hybrid central giant cell granuloma and central odontogenic fibroma-like lesions of the jaws.Histopathology 1997, 30:165–171. 10.1046/j.1365-2559.1997.d01-585.x
Kawakami T, Antoh M, Minemura T: Giant cell reaction to ameloblastoma: an immunohistochemical and ultrastructural study of a case.J Oral Maxillofac Surg 1989, 47:737–741. 10.1016/S0278-2391(89)80017-5
Richard BM, Thyveetil M, Sharif H, Athanasou NA: Ameloblastoma with stromal multinucleated giant cells.Histopathology 1994, 25:497–499. 10.1111/j.1365-2559.1994.tb00015.x
Peñarrocha-Diago MA, Cervera-Ballester J, Maestre-Ferrín L, Peñarrocha-Oltra D: Peripheral giant cell granuloma associated with dental implants: clinical case and literature review.J Oral Implantol 2012,38(S1):527–532. 10.1563/AAID-JOI-D-11-00143
Galindo-Moreno P, Hernández-Cortes P, Rios R, Sanchez-Fernández E, Camara M, OValle F: Immunophenotype of dental implant-associated peripheral giant cell reparative granuloma in a representative case report.J Oral Implantol 2013. [Epub ahead of print]
Olmedo DG, Paparella ML, Brandizzi D, Cabrini RL: Reactive lesions of peri-implant mucosa associated with titanium dental implants: a report of 2 cases.Int J Oral Maxillofac Surg 2010, 39:503–507. 10.1016/j.ijom.2009.11.007
Acknowledgment
We thank Dr Komal Koli for taking illustrative photographs and producing the histopathology micrographs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EIO saw and managed our patient, and contributed to drafting the manuscript; NV performed the excisional biopsy of the lesion and reviewed the histopathologic diagnosis; MS saw and managed our patient; GF supervised management of our patient; CDJ took clinical photographs of the lesion and X-rays, and supervised the management of our patient; KUEO read and interpreted the histopathology report, drafted the manuscript and edited the final draft. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ogbureke, E.I., Vigneswaran, N., Seals, M. et al. A peripheral giant cell granuloma with extensive osseous metaplasia or a hybrid peripheral giant cell granuloma-peripheral ossifying fibroma: a case report. J Med Case Reports 9, 14 (2015). https://doi.org/10.1186/1752-1947-9-14
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1752-1947-9-14