Abstract
Background
This study measured lymphocyte mitochondrial O2 consumption (cellular respiration) in children with trisomy 21.
Methods
Peripheral blood mononuclear cells were isolated from whole blood of trisomy 21 and control children and these cells were immediately used to measure cellular respiration rate. [O2] was determined as a function of time from the phosphorescence decay rates (1/τ) of Pd (II)-meso-tetra-(4-sulfonatophenyl)-tetrabenzoporphyrin. In sealed vials containing lymphocytes and glucose as a respiratory substrate, [O2] declined linearly with time, confirming the zero-order kinetics of O2 conversion to H2O by cytochrome oxidase. The rate of respiration (k, in μM O2 min-1), thus, was the negative of the slope of [O2] vs. time. Cyanide inhibited O2 consumption, confirming that oxidation occurred in the mitochondrial respiratory chain.
Results
For control children (age = 8.8 ± 5.6 years, n = 26), the mean (± SD) value of k c (in μM O2 per min per 107 cells) was 1.36 ± 0.79 (coefficient of variation, Cv = 58%; median = 1.17; range = 0.60 to 3.12; -2SD = 0.61). For children with trisomy 21 (age = 7.2 ± 4.6 years, n = 26), the values of k c were 0.82 ± 0.62 (Cv = 76%; median = 0.60; range = 0.20 to 2.80), p<0.001. Similar results (p<0.000) were obtained after excluding the five trisomy 21 children with elevated serum TSH (values >6.1 mU/L). Fourteen of 26 (54%) children with trisomy 21 had k c values of 0.20 to 0.60 (i.e., <−2SD). The values of k c positively correlated with body-mass index (BMI, R >0.302), serum creatinine (R >0.507), blood urea nitrogen (BUN, R >0.535) and albumin (R >0.446).
Conclusions
Children with trisomy 21 in this study have reduced lymphocyte bioenergetics. The clinical importance of this finding requires further studies.
Similar content being viewed by others
Background
Trisomy 21 is the most common chromosomal anomaly worldwide, affecting about 1 in 700 newborns [1]. These individuals typically have low resting metabolic rates [2] and are particularly susceptible to infections [3] and hypothyroidism [4]. Moreover, defects in the inner mitochondrial membrane potential [5] and mitochondrial respiratory chain enzymes are documented in these patients [6, 7]. Mitochondrial disturbances, increased oxidative stress and apoptosis have been described in the neurons, predisposing to precocious Alzheimer’s disease [8]. Alterations in metabolic enzymes (e.g., monoamine oxidase-B, cytochrome oxidase, isocitrate dehydrogenase and glutamate dehydrogenase) have been also linked to impaired energy metabolism in trisomy 21 children [9]. Calcium levels are lower than in control children, which may alter cellular signaling [10].
Increased congenital heart disease and other major anomalies are exceptionally frequent in children with trisomy 21. It is not known whether these defects are linked to the biological impairments described above.
The use of the phosphorescence oxygen analyzer to measure lymphocyte respiration was recently reported. Lymphocytes were shown to be suitable for screening of certain mitochondrial disorders [11]. These methodologies were used to measure lymphocyte respiration rates in children with trisomy 21 and compare them with rates in children without this disorder.
Methods
Reagents and solutions
Pd (II) complex of meso-tetra-(4-sulfonatophenyl)-tetrabenzoporphyrin was purchased from Porphyrin Products (Logan, UT). Glucose oxidase (powder from Aspergillus niger), D (+) glucose anhydrous, Histopaque-1077 and remaining reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Pd phosphor solution (2.5 mg/ml = 2 mM) was prepared in distilled water (dH2O) and stored at −20°C. Glucose oxidase solution was prepared in dH2O (10 mg/mL) and stored at −20°C. Sodium cyanide (NaCN) solution (1.0 M) was prepared in dH2O; the pH was adjusted to ~7.0 with 12N HCl and stored at −20°C. Phosphate-buffered saline (PBS) containing glucose (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4 and 10 mM glucose; pH 7.4) was stored at 4°C.
Subjects
Venous blood samples (5 to 8 mL) were collected in heparin tubes and processed in <2 hr for peripheral blood mononuclear cell (PBMC) isolation and O2 measurement. Blood was also collected from age- and gender-matched healthy controls. All trisomy 21 participants attended the outpatient facilities at Tawam and Al Ain Hospitals (Al Ain city, Abu Dhabi) for routine visits. All control participants were healthy children who had no medical complaints.
The study was approved by the institutional review board for protection of human subjects. Informed consent was obtained for each participating subject.
PBMC isolation
Plasma was collected from blood samples by centrifugation and possessed for Comprehensive Metabolic Panel and lipid profile. The samples were then diluted with equal volume of phosphate-buffered saline (PBS) containing 10 mM glucose and gently layered on the top of 10 mL Histopaque-1077. The mixtures were centrifuged at 15°C, 400 xg for 30 min. Collected PBMC were diluted with the same solution and re-centrifuged as above. The pellets were suspended in PBS, 10 mM glucose, 3 μM Pd phosphor and 0.5% fat-free bovine serum albumin for O2 measurements at 37°C. Cell count and viability were determined by light microscopy, using a hemocytometer under standard trypan blue staining conditions. Only trypan blue-negative cells (>95%) were counted.
Oxygen instrument
A phosphorescence oxygen analyzer that measures dissolved O2 in solutions as function of time was used to determine the rate of PBMC respiration [12, 13]. This method is based on the principle that O2 quenches the phosphorescence of a palladium phosphor [14].
The Pd (II) derivative of meso-tetra-(4-sulfonatophenyl)-tetrabenzoporphyrin had an absorption maximum at 625 nm and a phosphorescence emission maximum at 800 nm. Samples were exposed to light flashes (10 per sec) from a pulsed light-emitting diode array with a peak output at 625 nm. Emitted phosphorescent light was detected by a Hamamatsu photomultiplier tube after first passing it through a wide-band interference filter centered at 800 nm. Amplified phosphorescence was digitized at 1–2 MHz using an analog/digital converter (PCI-DAS 4020/12 I/O Board) with 1 to 20 MHz outputs.
The phosphorescence decay rate (1/τ) was characterized by a single exponential; I = Ae-t/τ, where I = Pd phosphor phosphorescence intensity. The values of 1/τ were linear with dissolved O2: 1/τ = 1/τo + k q [O2, where 1/τ = the phosphorescence decay rate in the presence of O2, 1/τo = the phosphorescence decay rate in the absence of O2, and k q = the second-order O2 quenching rate constant in sec-1 μM-1 (14). For calibration, the reaction contained PBS, 3 μM Pd phosphor, 0.5% fat-free albumin, 50 μg/mL glucose oxidase and various concentrations of β-glucose [11].
Cellular respiration was measured at 37°C in 1.0-mL sealed vials. Mixing was carried out with the aid of parylene-coated stirring bars. The respiratory substrates were endogenous metabolic fuels supplemented with glucose.
Statistical analysis
The data are summarized by arithmetic mean and standard deviation. Mann–Whitney U test was used for nonparametric values. P<0.05 was considered significant.
Results
In cell suspensions sealed from air, [O2] decreased linearly with time, indicating the kinetics of mitochondrial O2 consumption was zero-order. The rate of respiration (k, in μM O2/min) was thus the negative of the slope d[O2]/dt. Cyanide markedly inhibited respiration (≥96%), confirming O2 was consumed mainly by the mitochondrial respiratory chain.
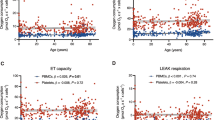
Lymphocyte respiration was measured in 26 children with trisomy 21 and 26 control children. Representative O2 runs are shown in Figure 1a-b. For trisomy 21 children, the rate of respiration (k c , in μM O2 per min per 107 cells, mean ± SD, n = 26) was 0.82 ± 0.62 (median = 0.60; range = 0.20 to 2.80), Table 1. The values of k c for control children (n = 26) were 1.36 ± 0.79 (median = 1.17; range = 0.60 to 3.12; -2SD = 0.61). The p value for k c between trisomy 21 and control children was <0.001, Figure 2. Similar results with higher significance (p<0.000) were obtained after excluding the five children with trisomy 21 and elevated serum TSH (values >6.1 mU/L). Fourteen of 26 (54%) children with trisomy 21 had k c values of 0.20 to 0.60 (<−2SD).
Representative O 2 runs for lymphocyte respiration in a 15-year-old male with trisomy 21 (Panel a, Subject 8 in Table 1 ) and control subject (Panel b). The lines are best linear fits (R 2 >0.830). The additions of 10 mM NaCN and 50 μg/mL glucose oxidase are shown.
In children with trisomy 21 and normal TSH (n = 21), the k c value did not correlate with the TSH level (R 2 >0.072, Figure 3a). By contrast, in children with trisomy 21 and abnormal lymphocyte respiration (k c < 0.61, n = 14), the k c value correlated with the TSH level (R 2 >0.225, R >0.474, Figure 3b).
Lymphocyte respiration in children with trisomy 21 as a function of serum TSH. Panel a: Circles, children (n = 21) with trisomy 21 and normal TSH (levels ≤5.3 mU/L; line is the best linear fit, R 2 > 0.0727); diamonds, children (n = 5) with trisomy 21 and elevated TSH (levels 7.7 to 13.2 mU/L). Panel b: Children (n = 14) with trisomy 21 and abnormal (low) rate of respiration (k c < 0.60 μM O2 per min per 107 cells). The horizontal line reflects upper limit of normal TSH (<6.1 mU/L, please see footnote to Table 1).
Five children with trisomy 21 had elevated TSH levels (>6.1 mIU/L). Their median TSH was 12.6 mIU/L (range, 6.4 to 13.2) and median k c was 0.7 μM O2 per min per 107 cells (range, 0.2 to 2.8). Subject 8 (15-year-old adolescent male) had a TSH level of 13.2 mIU/L and a k c values of 2.8 μM O2 per min per 107 cells (Table 1 and Figure 1a).
There were 8 children with trisomy 21 and congenital heart disease. Their median k c value was 0.6 μM O2 per min per 107 cells (range, 0.2 to 1.6), and did not significantly differ from the remaining children (p = 0.238).
In children with trisomy 21, the k c positively correlated with BMI (R >0.302, Figure 4a), serum creatinine (R >0.507), BUN (R >0.535) and albumin (R >0.446, Figure 4b), Table 2.
Discussion
The rates of lymphocyte respiration in the children with trisomy 21 were slower than in the control group (Figure 2). These differences could reflect a relatively lower rate of mitochondrial energy conversion in trisomy 21 children that may be linked to some pathological findings pertinent to this disorder, such as defects in the inner mitochondrial membrane [5, 8].
The mechanism for slower rates of lymphocyte respiration in children with trisomy 21 could be multi-factorial. For example, the thyroid hormone is a well known regulator of the rate of metabolism; and hypothyroidism is common in children with trisomy 21. As shown in Figure 3b, high TSH (low or ineffective thyroxin) may contribute to the slower lymphocyte respiration in some children. Thus, thyroxin replacement is expected to improve lymphocyte respiration in those with hypothyroidism.
Of note, normal TSH values for children 2 to 7 years of age are 0.10 to 5.9 mU/L (mean = 2.2 mU/L) and for children 9 to 16 years of age are 0.20 to 6.1 mU/L (mean = 2.3 mU/L). Using these cutoffs, lymphocyte respiration was found to be higher in euthyroid trisomy 21 children than those with hypothyroidism.
Body-mass index, protein metabolism (BUN, total protein and albumin), and serum creatinine positively correlated with rates of lymphocyte respiration, but only in trisomy 21 children (Table 2 and Figure 4a-b). As previously reported, protein metabolism (proteolysis, oxidation and synthesis) is linked to obesity [15, 16], a finding that is common in children with trisomy 21.
Close correlations were documented between cerebral O2 consumptions and mental function, including depression and dementia [17, 18]. It is unknown if our finding of slower lymphocyte respiration in trisomy 21 children is applicable to other organs. Nevertheless, our findings are consistent with the recent reports on mitochondrial disturbances in those with trisomy 21 [19–23]. Decreased basal 3'-5'-cyclic adenosine monophosphate, increased reactive oxygen species and impaired NADH:ubiquinone reductase (complex I of the respiratory chain) were noted in fibroblasts from those with trisomy 21 [20].
Limitations of the study
No study was found in the literature that addressed lymphocyte respiration in children with trisomy 21. Additional studies are needed in a larger population.
Conclusions
Children with trisomy 21 in this study have lower lymphocyte bioenergetics, a finding that is consistent with the known mitochondrial disturbances in these children. The clinical significance implication of this finding requires further studies.
References
Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, Correa A: Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006 birth defects. Res A Clin Mol Teratol. 2010, 88: 100-1016.
Luke A, Roizen NJ, Sutton M, Shoeller DA: Energy expenditure in children with Down syndrome: Correcting metabolic rate for movement. J Pediatr. 1994, 125: 829-838. 10.1016/S0022-3476(06)80193-9.
Doglus S: Down syndrome: Immunologic and Epidemiologic association-Enigmas remain. J Pediatr. 2005, 147: 724-725.
Murdoch JC, Ratcliffe WA, McLarty DG, Rodger JC, Ratcliffe JG: Thyroid function in adults with Down's syndrome. J Clin Endocrinol Metab. 1977, 44: 453-458. 10.1210/jcem-44-3-453.
Abu Faddan N, Sayed D, Ghaleb F: T-lymphocytes apoptosis and mitochondrial membrane potential in Down's syndrome. Fetal and Pediatric Pathology. 2011, 30: 45-52. 10.3109/15513815.2010.505626.
Baracca A, Sgarbi G, Solaini G, Lenaz G: Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F0 during ATP synthesis. Biochim Biophys Acta. 2003, 1606: 137-146. 10.1016/S0005-2728(03)00110-5.
Kirkinezos IG, Moraes CT: Reactive oxygen species and mitochondrial diseases. Seminars in Cell & Developmental Biology. 2001, 12: 449-457. 10.1006/scdb.2001.0282.
Sureda FX, Escubedo E, Gabriel C, Comas J, Camarasa J, Camins A: Mitochondrial membrane potential measurement in rat cerebellar neurons by flow cytometry. Cytometry. 1997, 28: 74-80. 10.1002/(SICI)1097-0320(19970501)28:1<74::AID-CYTO9>3.0.CO;2-H.
Prince J, Jia S, Båve U, Annerén G, Oreland L: Mitochondrial enzyme deficiencies in Down's syndrome. J Neural Transm Park Dis Dement Sect. 1994, 8: 171-181. 10.1007/BF02260938.
More R, Amir N, Meyer S, Kopolovic J, Yarom R: Platelet abnormalities in Down's syndrome. Clin Genet. 1982, 22: 128-136.
Al-Jasmi F, Penefsky HS, Souid A-K: The phosphorescence oxygen analyzer as a screening tool for disorders with impaired lymphocyte bioenergetics. Mol Genet Metab. 2011, 104: 529-536. 10.1016/j.ymgme.2011.09.023.
Shaban S, Marzouqi F, Al Mansouri A, Penefsky HS, Souid AK: Oxygen measurements via phosphorescence. Comput Methods Programs Biomed. 2010, 100: 265-268. 10.1016/j.cmpb.2010.04.009.
Souid AK, Tacka KA, Galvan KA, Penefsky HS: Immediate effects of anticancer drugs on mitochondrial oxygen consumption. Biochem Pharmacol. 2003, 66: 977-987. 10.1016/S0006-2952(03)00418-0.
Lo L-W, Koch CJ, Wilson DF: Calibration of oxygen-dependent quenching of the phosphorescence of Pd-meso-tetra (4-carboxyphenyl) porphine: A phosphor with general application for measuring oxygen concentration in biological systems. Anal Biochem. 1996, 236: 153-160. 10.1006/abio.1996.0144.
Solini A, Bonora E, Bonadonna R, Castellino P, DeFronzo RA: Protein metabolism in human obesity: relationship with glucose and lipid metabolism and with visceral adipose tissue. J Clin Endocrinol Metab. 1997, 82: 2552-2558. 10.1210/jc.82.8.2552.
Jensen MD, Haymond MW: Protein metabolism in obesity: effects of body fat distribution and hyperinsulinemia on leucine turnover. Am J Clin Nutr. 1991, 5: 172-176.
Lassen NA, Christensen S, Hoedt-Rasmussen K, Stewart BM: Cerebral oxygen consumption in Down's syndrome. Arch Neurol. 1966, 15: 595-602. 10.1001/archneur.1966.00470180035004.
Garfunkel JM, Baird HW, Ziegler J: The relation of oxygen consumption to cerebral function activity. J Pediatr. 1954, 44: 64-72. 10.1016/S0022-3476(54)80093-X.
Pagano G, Castello G: Oxidative stress and mitochondrial dysfunction in Down syndrome. Adv Exp Med Bio. 2012, 724: 291-299. 10.1007/978-1-4614-0653-2_22.
Valenti D, Manente GA, Moro L, Marra E, Vacca RA: Deficit of complex I activity in human skin fibroblasts with chromosome 21 trisomy and overproduction of reactive oxygen species by mitochondria: involvement of the cAMP/PKA signaling pathway. Biochem J. 2011, 435: 679-688. 10.1042/BJ20101908.
Infantino V, Castegna A, Iacobazzi F, Spera I, Scala I, Andria G, Iacobazzi V: Impairment of methyl cycle affects mitochondrial methyl availability and glutathione level in Down's syndrome. Mol Genet Metab. 2011, 102: 378-382. 10.1016/j.ymgme.2010.11.166.
Pallardó FV, Lloret A, Lebel M, d'Ischia M, Cogger VC, Le Couteur DG, Gadaleta MN, Castello G, Pagano G: Mitochondrial dysfunction in some oxidative stress-related genetic diseases: Ataxia-Telangiectasia, Down Syndrome, Fanconi Anemia and Werner Syndrome. Biogerontology. 2010, 11: 401-419. 10.1007/s10522-010-9269-4.
Valenti D, Tullo A, Caratozzolo MF, Merafina RS, Scartezzini P, Marra E, Vacca RA: Impairment of F1F0-ATPase, adenine nucleotide translocator and adenylate kinase causes mitochondrial energy deficit in human skin fibroblasts with chromosome 21 trisomy. Biochem J. 2010, 431: 299-310. 10.1042/BJ20100581.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2431/12/193/prepub
Acknowledgements
We thank Mr. Pramdan for running the experiments. We are grateful to Emirates Foundation for their fund support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EHA and AKS designed the study, carried out the analysis, interpreted the data and wrote the manuscript. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Aburawi, E.H., Souid, AK. Lymphocyte respiration in children with Trisomy 21. BMC Pediatr 12, 193 (2012). https://doi.org/10.1186/1471-2431-12-193
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2431-12-193