Abstract
Background
Diabetic patients have a higher risk of bladder cancer and benign prostatic hyperplasia (BPH). Theoretically, BPH patients may have an increased risk of bladder cancer because residual urine in the bladder surely increases the contact time between urinary excreted carcinogens and the urothelium. However, whether BPH increases bladder cancer risk in patients with type 2 diabetes has not been studied.
Methods
The reimbursement databases of all Taiwanese diabetic patients under oral anti-diabetic agents or insulin from 1996 to 2009 were retrieved from the National Health Insurance. An entry date was set at 1 January 2006 and a total of 547584 men with type 2 diabetes were followed up for bladder cancer incidence until the end of 2009. Incidences of bladder cancer for BPH by status and by duration were calculated and adjusted hazard ratios (95% confidence intervals) were estimated by Cox regression. The effects of diabetes duration and medications used for diabetic control in relation with bladder cancer risk were also evaluated by Cox regression in BPH men.
Results
The incidences were 258.77 and 69.34 per 100,000 person-years for patients with and without BPH, respectively, adjusted hazard ratio 1.794 (1.572, 2.047). For BPH patients, those who underwent surgical procedures for BPH had a higher incidence than those who did not (355.45 vs. 250.09 per 100,000 person-years), respective adjusted hazard ratios: 2.459 (1.946, 3.109) and 1.709 (1.492, 1.958). The significantly higher risk could be demonstrated for BPH of any duration: respective adjusted hazard ratios 1.750 (1.430, 1.605), 1.844 (1.543, 2.203), 2.011 (1.680, 2.406) and 1.605 (1.341, 1.921) for BPH <1, 1–3, 3–5 and ≥5 years versus patients without BPH. Sensitivity analyses for patients aged ≥60 years and after excluding BPH patients with surgical procedures or without surgical procedures, respectively, yielded similar results. In BPH men, diabetes duration was not significantly related with bladder cancer; but metformin was consistently associated with a significantly lower risk, with adjusted hazard ratio of 0.719 (0.590, 0.875) for all ages and 0.742 (0.604, 0.912) for age ≥60 years.
Conclusions
BPH is a significant risk factor for bladder cancer in men with type 2 diabetes. Metformin may protect against bladder cancer in BPH men.
Similar content being viewed by others
Background
Benign prostatic hyperplasia (BPH) is a common urological disorder in men. Its prevalence increases with age and may affect 3 of 4 men in their sixties [1–3]. BPH may cause gradual obstruction to the bladder outflow, leading to progressive severity in lower urinary tract symptoms such as frequency, urgency, nocturia, incomplete voiding and weak urinary stream.
Residual urine in the bladder in patients with BPH surely increases the contact time between urinary excreted carcinogens and the urothelium. Therefore, theoretically, patients with BPH may have an increased risk of bladder cancer. An animal study conducted in female Wistar rats supported such a hypothesis. Matsumoto et al. found that surgically induced partial bladder outlet obstruction resulted in a greater incidence of bladder cancer induced by the carcinogen n-butyl-n-butanol nitrosamine [4].
Human studies investigating the link between BPH and bladder cancer are still sparse. Two early case–control studies suggested an increased risk [5, 6]. A recent cohort study recruiting 79280 Swedish men hospitalized for BPH from 1964 to 1983 and following the patients until 1989 concluded that the overall risk of bladder cancer was not increased with BPH, but patients who underwent transurethral resection of the prostate had a significantly 47% higher risk [7].
On the other hand, diabetes may affect the lower urinary tract symptoms/functions [8–13], and patients with type 2 diabetes may have a higher risk of BPH [3, 10, 12, 14–17]. A study estimated that men with higher fasting glucose (>110 mg/dl vs. 110 mg/dl or less) or with a diagnosis of diabetes may have a significantly 3-fold and 2.3-fold higher risk of BPH, respectively [14]. Actually, type 2 diabetes and BPH share several common risk factors including aging, insulin resistance and obesity [3]. Studies suggested that type 2 diabetes, obesity and metabolic syndrome may all affect the growth of BPH. For example, an earlier study showed that the annual BPH growth rate for patients without and with type 2 diabetes was 0.928 ml/year and 1.385 ml/year, respectively [17]. The Baltimore Longitudinal Study of Aging suggested that prostate volume increased 0.41 ml with each 1-kg/m2 increment of body mass index, and there was a 3.5-fold higher risk of BPH comparing a body mass index of ≥35 kg/m2 to that of <25 kg/m2[14]. A study in Turkish men showed that the growth rate of total prostate in BPH patients with and without metabolic syndrome was 1.0 ml/year and 0.64 ml/year, respectively (P = 0.018) [18]. Therefore, the etiology underlying the development of metabolic syndrome including diabetes and obesity may also lead to the growth of prostate.
The number of diabetic patients has been increasing dramatically all over the world in recent decades [19], and this is especially remarkable in the Asian populations [20]. It is estimated that the global number of diabetic patients will increase from 171 million in 2000 to 366 million in 2030; and most of these patients will be seen in Asian countries, with an estimated number of 75 million in 2000 and 180 million in 2030 [19]. Recently, patients with type 2 diabetes have been shown to carry a higher risk of bladder cancer [21–24] and the use of some anti-diabetic agents (e.g. pioglitazone) might be associated with an increased risk [25–28]. However, whether BPH can be a risk factor for bladder cancer in the diabetic patients has not been studied. An elucidation of the association between BPH and bladder cancer is not only an issue of scientific interest, it is also important for the planning and implementing programs for the prevention of bladder cancer, either by reducing the incidence of diabetes and BPH or by early treatment of these diseases. Therefore, the purpose of the present study was to evaluate, in men with type 2 diabetes, whether BPH could be a risk factor for bladder cancer.
Methods
Since March 1995 a compulsory and universal system of health insurance (the so-called National Health Insurance, NHI) was implemented in Taiwan. All contracted medical institutes must submit computerized and standard claim documents for reimbursement. More than 99% of citizens are enrolled in the NHI, and >98% of the hospitals nationwide are under contract with the NHI. The average number of annual physician visits in Taiwan is one of the highest around the world, at approximately 15 visits per year per capita in 2009.
The National Health Research Institute is the only institute approved, as per local regulations, for handling the NHI reimbursement databases for academic research. The databases contain detailed records on every visit for each patient, including outpatient visits, emergency department visits and hospital admission. The databases also include principal and secondary diagnostic codes, prescription orders, and claimed expenses. The identification information of the individuals was scrambled for the protection of privacy. Diabetes was coded 250.1-250.9, BPH 600, and bladder cancer 188, based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).
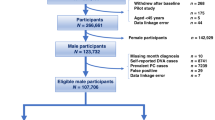
We first retrieved the databases of all patients who had been diagnosed as having diabetes and under treatment with either oral anti-diabetic agents or insulin during the period of 1996–2009 from the whole nation (n = 1789776). The selected entry date was 1 January 2006. After excluding patients who had a diagnosis of diabetes after the year 2006 (n = 342351), patients who held a Severe Morbidity Card as having type 1 diabetes (n = 7120, in Taiwan, patients with type 1 diabetes were issued a so-called “Severe Morbidity Card” after certified diagnosis and they were waived for much of the co-payments), patients having a diagnosis of bladder cancer before 2006 (n = 9555), those who died (n = 96320) or withdrew from the NHI (n = 12502) before entry date, duplicated identification number (n = 106), unclear information on date of birth or sex (n = 5122), and diabetic patients without any reimbursement record after the entry date (n = 235746), a total of 1094404 patients with a diagnosis of type 2 diabetes and under therapy with oral anti-diabetic agents or insulin were recruited. A further exclusion of the female sex yielded 547584 men with type 2 diabetes for the present study.
All comorbidities and covariates were determined as a status/diagnosis before the entry date. The ICD-9-CM codes for the comorbidities were [21, 29, 30]: nephropathy 580–589, urinary tract disease 590–599, hypertension 401–405, chronic obstructive pulmonary disease (a surrogate for smoking) 490–496, cerebrovascular disease 430–438, ischemic heart disease 410–414, peripheral arterial disease 250.7, 785.4, 443.81 and 440–448, eye disease 250.5, 362.0, 369, 366.41 and 365.44, dyslipidemia 272.0-272.4, heart failure 398.91, 402.11, 402.91, 404.11, 404.13, 404.91, 404.93 and 428, obesity 278, alcohol-related diagnosis 291, 303, 535.3, 571.0, 571.1, 571.2, 571.3 and 980.0, non-alcohol-related chronic liver disease 570–573, 070 and 571.4 (excluding 571.0, 571.1, 571.2 and 571.3), and cancer other than bladder cancer 140–208 (excluding 188). Medications included rosiglitazone, pioglitazone, sulfonylurea, meglitinide, metformin, acarbose, insulin, statin, fibrate, angiotensin-converting enzyme inhibitor and/or angiotensin receptor blocker, calcium channel blocker, non-steroidal anti-inflammatory drugs, alpha-blockers, 5-alpha reductase inhibitors, clopidogrel, ticlopidine, dipyridamole, cyclophosphamide and diuretics. Baseline characteristics between patients with and without BPH were compared by Chi-square test.
The incidence density of bladder cancer was calculated for BPH by status and by duration of BPH diagnosis (<1 year, 1–3 years, 3–5 years and ≥5 years) in patients of all ages and in patients aged ≥60 years, respectively. Patients with BPH were further categorized into two subgroups: with and without receiving surgical procedures for the treatment of BPH, including transurethral incision of the prostate, transurethral resection of the prostate, suprapubic prostatectomy or retropubic prostatectomy. The numerator for the incidence was the number of patients with incident bladder cancer during the 4-year follow-up from 1 January 2006 until 31 December 2009, and the denominator was the person-years of follow-up.
To compare whether patients with BPH had a higher probability of visits to the urologists or receiving laboratory examinations that might potentially lead to the diagnosis of bladder cancer, the frequencies of these items between patients with and without BPH were analyzed by Chi square test, in all patients and in patients aged ≥60 years, respectively. Laboratory examinations included serum tumor markers (including carcinoembryonic antigen, carbohydrate antigen 19–9, carbohydrate antigen 125 and tissue polypeptide antigen), urine cytology, cystoscopy, urinalysis and bladder ultrasonography. The nuclear matrix protein-22 and the bladder tumor associated antigen tests for screening bladder cancer were not reimbursed by the NHI; and therefore they could not be considered in the analyses.
Cox proportional hazards regression was performed to estimate the hazard ratios for bladder cancer for patients with BPH vs. patients without BPH. The following models were created: 1) BPH vs. no BPH; 2) BPH without surgical procedures, BPH with surgical procedures vs. no BPH; and 3) BPH by duration (<1 year, 1–3 years, 3–5 years and ≥5 years) vs. no BPH. For sensitivity analyses, the Cox models were also created after excluding patients with surgical procedures and without surgical procedures, respectively, from the analyses. All models were created for all ages and age ≥60 years, respectively. Age, diabetes duration, comorbidities, medications and potential detection examinations were all adjusted for in the models.
To further evaluate the possible effects of diabetes duration and medications used for diabetic control on the risk of bladder cancer among the BPH men, additional Cox regression models were created for the diabetic men with BPH for all ages and for age ≥60 years, respectively. Age, comorbidities, medications other than anti-diabetic drugs and potential detection examinations were all adjusted for in these models.
Analyses were conducted using SAS statistical software, version 9.1 (SAS Institute, Cary, NC). P < 0.05 was considered statistically significant.
Results
Table 1 compares the baseline characteristics between patients with BPH and those without. Patients with BPH were older and had longer diabetes duration, higher rates of comorbidities (except obesity and alcohol-related diagnosis), and higher rates of using medications and other cancer.
Table 2 shows the crude incidence of bladder cancer with regards to BPH status or duration. The incidence was 69.34 per 100,000 person-years for patients without BPH and was 258.77 per 100,000 person-years for patients with BPH. For BPH patients, those who underwent a surgical procedure had a higher incidence than those who had not received a surgical procedure (355.45 vs. 250.09 per 100,000 person-years). The incidence seemed to increase with longer duration of BPH and peaked at BPH duration of 3–5 years, especially in those who underwent a surgical procedure. The findings were similar in the analyses for those aged ≥60 years.
Table 3 compares the frequency of visits to urologists and laboratory examinations that might potentially lead to the diagnosis of bladder cancer in patients with and without BPH. It is true that patients with BPH had a significantly higher probability of potential detection bias.
The hazard ratios with regards to BPH by status or duration are presented in Table 4. Except for BPH by duration <1 year in the sensitivity analyses after excluding BPH patients without a surgical procedure from the analyses, all hazard ratios showed a significantly higher risk of bladder cancer associated with BPH. For analyses in all patients of all ages, patients with BPH had a significantly higher risk, with hazard ratio (95% confidence interval): 1.794 (1.572, 2.047). BPH patients with a surgical procedure had a larger magnitude of hazard ratio than those without: 2.459 (1.946, 3.109) vs. 1.709 (1.492, 1.958). The significantly higher risk could be demonstrated in patients with BPH of any duration. The analyses were similar in the sensitivity analyses, but the magnitudes of hazard ratios were larger in patients with a surgical procedure than those without a surgical procedure in any specific BPH duration (except for the category with BPH duration <1 year).
Table 5 shows the adjusted hazard ratios for bladder cancer with regards to diabetes duration and medications used for diabetic control in the diabetic men with BPH. It was noted that diabetes duration was not significantly related to bladder cancer in the BPH men; and that among all anti-diabetic drugs only metformin was significantly associated with a lower risk of bladder cancer in the BPH men.
Discussion
The present study strongly suggested that BPH is an important risk factor for bladder cancer in patients with type 2 diabetes (Tables 2 and 4). Furthermore, BPH patients who had received a surgical procedure might have a higher magnitude of hazard ratio than those without a surgical procedure (Tables 2 and 4), suggesting that patients with more severe clinical conditions of BPH might have a higher risk of bladder cancer. Although BPH patients were older, had higher prevalences of most comorbidities and medications used (Table 1) and a higher probability of detection bias (Table 3), the risk associated with BPH could not be ascribed to these confounders (Table 4). The effect of BPH on bladder cancer risk seemed to be independent of diabetes because none of the diabetes duration was significantly related with bladder cancer in the BPH men (Table 5). It was also observed that, in the BPH men, those who used metformin might have a significantly lower risk of bladder cancer than those who had never used metformin (Table 5), suggesting a potentially preventive effect of metformin against bladder cancer development.
A possible explanation for a link between BPH and bladder cancer is that the residual urine in the bladder in patients with BPH may increase the time of urothelial exposure to urinary excreted carcinogens. Such a hypothesis is strongly supported by the animal study by Matsumoto et al. because bladder cancer induced by the carcinogen n-butyl-n-butanol nitrosamine may also be aggravated by surgically induced bladder outlet obstruction in female Wistar rats [4]. Another circumstantial evidence supporting the hypothesis in humans came from prospective [31] and case–control [32] studies showing that high fluid intake, indicating less concentrated urine or more frequent micturition, was associated with a lower risk of bladder cancer.
Patients with BPH also have a significantly higher risk of chronic kidney disease, probably due to an obstructive uropathy [33]. Chronic kidney disease has been consistently proved to be a significant risk factor for bladder cancer in the Taiwanese population [21, 34]. Therefore, the increased risk of bladder cancer in patients with BPH could also be partially explained by the higher incidence of chronic kidney disease in these patients.
In the Swedish prospective follow-up study, there was a lack of an overall risk of bladder cancer associated with BPH [7], in contrary to a significant association in the present study (Tables 2 and 4). There were several possible explanations. First, the Swedish study used the bladder cancer incidence in the general population as referents for the calculation of the standardized incidence ratio. The referent groups would surely include the BPH patients in the study and also include other high risk patients like the diabetic patients who may have an increased risk of bladder cancer [21–24]. Second, the Swedish study could not consider the adjustment for potential confounders except for age. Furthermore, different ethnicities might also be a possible explanation. It should be pointed out that the Swedish study did show a significantly 2-fold higher risk of bladder cancer in a subgroup of the BPH men who underwent transurethral resection of the prostate and had genitourinary conditions such as urinary tract infection and stones [7]. This was consistent with the findings of the present study (Tables 2 and 4), and with our previous study showing a significantly higher risk of bladder cancer in patients with a history of urinary tract infection or stones [21].
Although the etiology of BPH remains unknown, metabolic disturbances may promote the growth of prostate. Obesity, insulin resistance with elevated insulin level, and higher serum concentration of insulin-like growth factor-I are associated with a significantly higher risk of BPH [3, 35, 36]. Obesity and diabetes are also associated with systemic inflammation and oxidative stress, which may promote the inflammatory processes in the prostate, leading to clinical development of BPH [3]. The link between BPH and some lifestyle risk factors of type 2 diabetes such as physical inactivity and dietary factors including increased total energy intake and less vegetables or vitamin D [3, 15] also strongly support the existence of some common underlying etiology for BPH and type 2 diabetes, which may well explain the observed association between BPH and type 2 diabetes.
Diabetic patients have an increased risk of bladder cancer [21–24]. The etiology for such a link has not yet been clarified. Some possible mechanisms have been proposed based on elevated blood glucose, insulin resistance, elevated levels of insulin and insulin-like growth factor-I, higher prevalences of neurogenic bladder and sympathetic nerve dysfunction in the diabetic patients [12]. Some recent studies also suggested a possible higher risk of bladder cancer associated with the use of pioglitazone in patients with type 2 diabetes [25–28]. The present study suggested another possible mechanism, which acts through BPH in the diabetic patients.
There are several important clinical implications from the present study. First, BPH-related bladder cancer can be preventable if BPH is prevented or adequately treated at its early stage. Second, in diabetic men with BPH, the use of metformin is potentially preventive for the development of bladder cancer. This finding surely provides a good rationale for the conduction of clinical trials evaluating the use of metformin for the prevention of bladder cancer among high-risk patients with BPH. Third, type 2 diabetes is increasing more remarkably in men than in women in the Taiwanese younger generation in recent 2 decades, probably due to the increasing epidemic of obesity in the young men [37, 38]. It is expected that the incidence of bladder cancer in the male population will be increasing in the future when these young and obese diabetic patients become older, because of the link between diabetes and BPH and the higher risk of bladder cancer related to BPH as shown in the present study. Therefore lifestyle modification to reduce the incidence of obesity, diabetes and metabolic syndrome is mandatory not only for preventing the development of BPH, but also for reducing the incidence of bladder cancer. Fourth, the potential confounding effect of BPH should not be neglected while evaluating the association between some medications and bladder cancer risk. For examples, most recent studies evaluating the risk of bladder cancer related to pioglitazone have not seriously considered such a potential confounding from BPH and therefore their conclusion of a positive link could be challenged with a lack of such an adjustment for BPH. Furthermore, an increased risk of bladder cancer has been warned for a new class of oral anti-diabetic agents being under consideration for approval for human use, i.e., the sodium-dependent glucose cotransporter 2 inhibitors with a novel mechanism of inhibiting glucose reabsorption from the kidney [39]. It is strongly suggested that the potential confounding effect of BPH on bladder cancer risk should be assessed during the evaluation for its approval.
Because all diabetic patients were treated with oral anti-diabetic medications or insulin at entry, the chance of misclassification of diabetes was actually very low. However, we could not exclude the possibility of misclassification of bladder cancer and BPH. The probability of misclassification of bladder cancer must be low, because labeled diagnoses should be printed out in all prescriptions handed to the patients. Mislabeling of a cancer diagnosis would not be acceptable to the patients when they saw the diagnosis. With regards to the misclassification of BPH, this might not occur in patients who had received a surgical procedure. Our analyses in different subgroups of the BPH patients (Tables 2 and 4) did not favor a possible impact resulting from such a misclassification. It should be stressed that underdiagnosis of BPH was possible, especially when it was in the early stage without remarkable symptoms. However, if BPH increased the risk of bladder cancer, a misclassification of patients with BPH in the referent group would only have underestimated the hazard ratios.
Because the databases were derived from the whole population and they spanned the whole period from the beginning of the NHI to the end of 2009, there was no concern of potential selection bias related to sampling error. The longitudinal nature of the study and the use of medical records reduced the likelihood of reverse causality and recall bias. However, it should also be mentioned that the local law restricts to retrieve only a certain percentage of the reimbursement data from the whole NHI databases for academic research. Therefore, it was not possible for the present study to include the detailed information on surgical procedures for bladder cancer and additional data from non-diabetic general population for more in-depth analyses on the use of total cystectomy as a surrogate for “invasive bladder cancer” and for a comparison of bladder cancer risk associated with BPH in non-diabetic Taiwanese men, respectively.
Smoking is an important risk factor for bladder cancer [40], but we did not have information of smoking for adjustment and could only consider surrogates that are highly related to smoking, such as chronic obstructive pulmonary disease, ischemic heart disease, cerebrovascular disease and peripheral arterial disease (Table 4). Theoretically, a confounder should be correlated simultaneously with both the exposure (BPH) and the outcome (bladder cancer), and it should not be an intermediate between exposure and outcome [41]. There is no evidence showing smoking as a determinant for BPH [2, 15, 16].
This study has several strengths. The databases included all claim records on outpatient visits, emergency department visits and hospital admission, and we caught the diagnoses from all sources. Cancer is considered a severe morbidity by the NHI and most medical co-payments can be waived. Furthermore, there is a low drug cost-sharing required by the NHI and patients with certain conditions such as low-income household, veterans or patients with prescription refills for chronic disease are exempted from the drug cost-sharing [42]. Therefore the detection rate of bladder cancer would not tend to differ among different social classes.
The study limitations included a lack of actual measurement data for confounders such as obesity, smoking, alcohol drinking, water intake, family history, lifestyle, diet, hair dye use, and some occupational exposure and genetic parameters. In addition, we did not have biochemical data for evaluating their impact. Another limitation is the lack of information on the grading and staging of bladder cancer.
Conclusions
This population-based cohort study in Taiwan suggests that BPH is a significant risk factor for bladder cancer in men with type 2 diabetes. Metformin may protect against bladder cancer in the BPH men. This commonly seen risk factor of BPH should not be neglected in future studies evaluating bladder cancer risk.
Abbreviations
- BPH:
-
Benign prostatic hyperplasia
- ICD-9-CM:
-
International Classification of Diseases, Ninth Revision, Clinical Modification
- NHI:
-
National Health Insurance.
References
Wei JT, Calhoun E, Jacobsen SJ: Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2008, 179 (5 Suppl): S75-S80.
Zhang Y, Zhu C, Curado MP, Zheng T, Boyle P: Changing patterns of bladder cancer in the USA: evidence of heterogeneous disease. BJU Int. 2012, 109: 52-56.
Parsons JK: Benign prostatic hyperplasia and male lower urinary tract symptoms: epidemiology and risk factors. Curr Bladder Dysfunct Rep. 2010, 5: 212-218. 10.1007/s11884-010-0067-2.
Matsumoto S, Shimizu N, Hanai T, Uemura H, Levin R: Bladder outlet obstruction accelerates bladder carcinogenesis. BJU Int. 2009, 103: 1436-1439. 10.1111/j.1464-410X.2008.08261.x.
Mommsen S, Sell A: Prostatic hypertrophy and venereal disease as possible risk factors in the development of bladder cancer. Urol Res. 1983, 11: 49-52.
Nakata S, Sato J, Ohtake N, Imai K, Yamanaka H: Epidemiological study of risk factors for bladder cancer. Hinyokika Kiyo. 1995, 41: 969-977. Japanese
Kang D, Chokkalingam AP, Gridley G, Nyren O, Johansson JE, Adami HO, Silverman D, Hsing AW: Benign prostatic hyperplasia and subsequent risk of bladder cancer. Br J Cancer. 2007, 96: 1475-1479. 10.1038/sj.bjc.6603730.
Sarma AV, Burke JP, Jacobson DJ, McGree ME, St Sauver J, Girman CJ, Lieber MM, Herman W, Macoska J, Montie JE, Jacobsen SJ: Associations between diabetes and clinical markers of benign prostatic hyperplasia among community-dwelling Black and White men. Diabetes Care. 2008, 31: 476-482.
Sarma AV, St Sauver JL, Hollingsworth JM, Jacobson DJ, McGree ME, Dunn RL, Lieber MM, Jacobsen SJ, Urologic Diseases in America Project: Diabetes treatment and progression of benign prostatic hyperplasia in community-dwelling black and white men. Urology. 2012, 79: 102-108. 10.1016/j.urology.2011.08.065.
Michel MC, Mehlburger L, Schumacher H, Bressel HU, Goepel M: Effect of diabetes on lower urinary tract symptoms in patients with benign prostatic hyperplasia. J Urol. 2000, 163: 1725-1729. 10.1016/S0022-5347(05)67529-5.
Boon TA, Van Venrooij GE, Eckhardt MD: Effect of diabetes mellitus on lower urinary tract symptoms and dysfunction in patients with benign prostatic hyperplasia. Curr Urol Rep. 2001, 2: 297-301. 10.1007/s11934-001-0067-z.
Sarma AV, Parsons JK, McVary K, Wei JT: Diabetes and benign prostatic hyperplasia/lower urinary tract symptoms–what do we know?. J Urol. 2009, 182 (6 Suppl): S32-S37.
Burke JP, Jacobson DJ, McGree ME, Roberts RO, Girman CJ, Lieber MM, Jacobsen SJ: Diabetes and benign prostatic hyperplasia progression in Olmsted County Minnesota. Urology. 2006, 67: 22-25. 10.1016/j.urology.2005.08.010.
Parsons JK, Carter HB, Partin AW, Windham BG, Metter EJ, Ferrucci L, Landis P, Platz EA: Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006, 91: 2562-2568. 10.1210/jc.2005-2799.
Parsons JK: Lifestyle factors, benign prostatic hyperplasia, and lower urinary tract symptoms. Curr Opin Urol. 2011, 21: 1-4. 10.1097/MOU.0b013e32834100c9.
Parsons JK: Modifiable risk factors for benign prostatic hyperplasia and lower urinary tract symptoms: new approaches to old problems. J Urol. 2007, 178: 395-401. 10.1016/j.juro.2007.03.103.
Hammarsten J, Högstedt B, Holthuis N, Mellström D: Components of the metabolic syndrome-risk factors for the development of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 1998, 1: 157-162. 10.1038/sj.pcan.4500221.
Ozden C, Ozdal OL, Urgancioglu G, Koyuncu H, Gokkaya S, Memis A: The correlation between metabolic syndrome and prostatic growth in patients with benign prostatic hyperplasia. Eur Urol. 2007, 51: 199-203. 10.1016/j.eururo.2006.05.040.
Wild S, Roglic G, Green A, Sicree R, King H: Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004, 27: 1047-1053. 10.2337/diacare.27.5.1047.
Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB: Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009, 301: 2129-2140. 10.1001/jama.2009.726.
Tseng CH: Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia. 2011, 54: 2009-2015. 10.1007/s00125-011-2171-z.
Tseng CH, Chong CK, Tseng CP, Chan TT: Age-related risk of mortality from bladder cancer in diabetic patients: a 12-year follow-up of a national cohort in Taiwan. Ann Med. 2009, 41: 371-379. 10.1080/07853890902729778.
Larsson SC, Orsini N, Brismar K, Wolk A: Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia. 2006, 49: 2819-2823. 10.1007/s00125-006-0468-0.
Arcidiacono B, Iiritano S, Nocera A, Possidente K, Nevolo MT, Ventura V, Foti D, Chiefari E, Brunetti A: Insulin resistance and cancer risk: an overview of the pathogenetic mechanisms. Exp Diabetes Res. 2012, 2012: 789174-
Tseng CH: Pioglitazone and bladder cancer in human studies: is it diabetes itself, diabetes drugs, flawed analyses or different ethnicities?. J Formos Med Assoc. 2012, 111: 123-131. 10.1016/j.jfma.2011.10.003.
Tseng CH: Pioglitazone and bladder cancer: a population-based study of Taiwanese. Diabetes Care. 2012, 35: 278-280. 10.2337/dc11-1449.
Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP, Vaughn DJ, Nessel L, Selby J, Strom BL: Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011, 34: 916-922. 10.2337/dc10-1068.
Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, Allemand H: Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012, 55: 1953-1962. 10.1007/s00125-012-2538-9.
Tseng CH: Diabetes and risk of prostate cancer: a study using the National Health Insurance. Diabetes Care. 2011, 34: 616-621. 10.2337/dc10-1640.
Tseng CH: Diabetes, metformin use, and colon cancer: a population-based cohort study in Taiwan. Eur J Endocrinol. 2012, 167: 409-416. 10.1530/EJE-12-0369.
Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Curhan GC, Willett WC, Giovannucci EL: Fluid intake and the risk of bladder cancer in men. N Engl J Med. 1999, 340: 1390-1397. 10.1056/NEJM199905063401803.
Jiang X, Castelao JE, Groshen S, Cortessis VK, Shibata DK, Conti DV, Gago-Dominguez M: Water intake and bladder cancer risk in Los Angeles County. Int J Cancer. 2008, 123: 1649-1656. 10.1002/ijc.23711.
Rule AD, Jacobson DJ, Roberts RO, Girman CJ, McGree ME, Lieber MM, Jacobsen SJ: The association between benign prostatic hyperplasia and chronic kidney disease in community-dwelling men. Kidney Int. 2005, 67: 2376-2382. 10.1111/j.1523-1755.2005.00344.x.
Chen CH, Shun CT, Huang KH, Huang CY, Yu HJ, Pu YS: Characteristics of female non-muscle-invasive bladder cancer in Taiwan: association with upper tract urothelial carcinoma and end-stage renal disease. Urology. 2008, 71: 1155-1160. 10.1016/j.urology.2007.11.140.
Hsing AW, Chua S, Gao YT, Gentzschein E, Chang L, Deng J, Stanczyk FZ: Prostate cancer risk and serum levels of insulin and leptin: a population-based study. J Natl Cancer Inst. 2001, 93: 783-789. 10.1093/jnci/93.10.783.
Chokkalingam AP, Gao YT, Deng J, Stanczyk FZ, Sesterhenn IA, Mostofi FK, Fraumeni JF, Hsing AW: Insulin-like growth factors and risk of benign prostatic hyperplasia. Prostate. 2002, 52: 98-105. 10.1002/pros.10096.
Tseng CH, Tseng CP, Chong CK, Huang TP, Song YM, Chou CW, Lai SM, Tai TY, Cheng JC: Increasing incidence of diagnosed type 2 diabetes in Taiwan: analysis of data from a national cohort. Diabetologia. 2006, 49: 1755-1760. 10.1007/s00125-006-0314-4.
Tseng CH: The epidemiologic transition of diabetes mellitus in Taiwan: Implications for reversal of female preponderance from a national cohort. Open Diabetes Journal. 2009, 2: 18-23. 10.2174/1876524600902010018.
US Food & Drug Administration, Endocrinologic & Metabolic Advisory Committee: Dapagliflozin (BMS-512148) EMDAC Background Document. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM262996.pdf (accessed 10 August 2011)
Jacobs BL, Lee CT, Montie JE: Bladder cancer in 2010: how far have we come?. CA Cancer J Clin. 2010, 60: 244-272. 10.3322/caac.20077.
van Stralen KJ, Dekker FW, Zoccali C, Jager KJ: Confounding. Nephron Clin Pract. 2010, 116: c143-c147. 10.1159/000315883.
Cheng TM: Taiwan's new national health insurance program: genesis and experience so far. Health Aff (Millwood). 2003, 22: 61-76. 10.1377/hlthaff.22.3.61.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/13/7/prepub
Acknowledgments
The author thanks the Department of Health (DOH89-TD-1035; DOH97-TD-D-113-97009) and the National Science Council (NSC 101-2314-B-002-117) of Taiwan for their continuous support on epidemiologic studies of diabetes. The study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes (Registered number 99274). The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health or National Health Research Institutes.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
No competing interests to declare.
Authors’ contributions
CH researched data and wrote manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Tseng, CH. Benign prostatic hyperplasia is a significant risk factor for bladder cancer in diabetic patients: a population-based cohort study using the National Health Insurance in Taiwan. BMC Cancer 13, 7 (2013). https://doi.org/10.1186/1471-2407-13-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-13-7