Abstract
Background
Bupleurum marginatum Wall. ex DC (Apiaceae) is a perennial herb widely used in traditional Chinese and Kampo medicine for the treatment of various infectious diseases. The biological activities of B. marginatum have not been fully investigated. This study aims to investigate the antitrypanosomal, antimicrobial and antiviral activities of methanol (ME) and dichloromethane (DCM) extracts of B. marginatum aerial parts and the ability of both extracts to inhibit the growth of different cancer cell lines.
Methods
Phytochemical characterization of the extracts was performed by LC-MS profiling. The antitrypanosomal activity was evaluated using the resazurin method. The antimicrobial activity was assessed using agar diffusion and microdilution methods, and the minimum inhibitory concentration (MIC) values were determined. The antiviral activity was determined for 6.25, 12.5, and 50 μg/mL doses using a plaque reduction assay. Cytotoxicity was investigated in eight cancer cell lines (Caco-2, CCL-81, CCRF-CEM, COS-7, HL-60, MIA PaCa-2, MCF-7, and PANC-1) using the MTT assay and the caspase 3/7 activity was determined over the range of 62.5–1000 μg/mL.
Results
Phytochemical analyses resulted in the characterization of 15 components, mainly flavonoids and lignans. The DCM extract showed significant antitrypanosomal activity (IC50: 36.21 μg/mL) and moderate activity against Streptococcus pyogenes (MIC value: 0.25 mg/mL). At a dose of 12.5 μg/mL, the DCM extract inhibited 73.6% of the plaque production by hepatitis A virus. CCRF-CEM cells were the most sensitive to both extracts (IC50: 12.5–22.7 μg/mL). The cytotoxicity was mediated by induction of apoptosis (19-fold increase in the cellular caspase 3/7 level after treatment with the DCM extract at 1 mg/mL).
Conclusions
ME and DCM extract of B. marginatum showed anti-infective and antiproliferative effects.
Similar content being viewed by others
Introduction
Plants have been the foundation of traditional medicinal systems in many countries for hundreds of years to prevent or treat ailments and health disorders. Despite the tremendous success in manufacturing synthetic, semisynthetic therapeutic agents or both, approximately 80% of the world population still relies on herbal medicines for their health care [1].
The family Apiaceae comprises more than 3500 species, of which 300 are medicinally active and have been used in folk medicine [2]. Among the Apiaceae genera, Bupleurum encompasses about 180–190 species [3]. Bupleurum marginatum Wall. ex DC is a perennial herb of up to 60 cm in height, with oblanceolate leaves and yellow umbel flowers, and used in Chinese medicine [4].
B. marginatum has been widely used in Europe, China, Korea, and Japan in the form of herbal teas, either alone or in combination with other ingredients, for treatment of the common cold and inflammation [5, 6]. In addition, it is employed to treat hepatitis, cancer, microbial infections, and fever associated with malaria [7–9].
The secondary metabolites of B. marginatum have been investigated previously, and various classes of compounds were identified. Specifically, saikosaponins were found in small amounts [10], while flavonoid glycosides and aglycones such as rutin, narcissin, isoquercetrin, isorhamnetin, and quercetin were commonly found in the plant [11]. In addition, lignans such as marginatoxin [11–13] and many phytosterols (stigmasterol, α-spinasterol, β-sitosterol, and daucosterol) have been described from both the aerial and subterranean parts [6, 12].
Our previous work in 2009 [14] investigated the antibacterial, antifungal, antioxidant, anti-inflammatory and cytotoxic activity of the essential oil from the B. marginatum aerial parts. To our knowledge, the biological activities of different extracts from B. marginatum have not been studied in detail.
This study aims to investigate the antitrypanosomal, antimicrobial and antiviral activities of methanol (ME) and dichloromethane (DCM) extracts of B. marginatum aerial parts and the ability of both extracts to inhibit the growth of different cancer cell lines.
Methods
Plant materials
The aerial parts of B. marginatum were kindly provided by Prof. Thomas Efferth (University of Mainz, Mainz, Germany). The identity of the plant was ascertained by one of the authors (MA) in our laboratory by DNA barcoding and confirmed morphologically at the Botanical Garden, Heidelberg University (Heidelberg, Germany). Voucher specimens of the plant material were deposited at the Department of Biology, Institute of Pharmacy and Molecular Biotechnology, Heidelberg University under accession number IPMB P7367.
Chemicals
Chemicals were purchased from AppliChem® (Darmstadt, Germany), Fluka® (Buchs, Switzerland), and Sigma® (Sternheim, Germany). The solvents used were of analytical grade unless otherwise mentioned, and were purchased from Merck® (Darmstadt, Germany), J.T. Backer® (Deventer, Netherlands), and Theo Seulberger® (Karlsruhe, Germany). Media and supplements for cell cultures were obtained from Gibco® (Invitrogen, Karlsruhe, Germany) and Greiner Labortechnik® (Frickenhausen, Germany).
Preparation of ME and DCM extracts
The ME and DCM extracts were prepared by refluxing 100 g of finely milled, air-dried aerial parts of B. marginatum with 1.5 L of methanol or dichloromethane, respectively, for 6 h. The extracts were filtered, dried by passage over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure at 40°C in a Büchi rotavapor R-200 with B-172 Büchi vacuum system (Büchi Labortechnik, Flawil, Switzerland). The dried extracts were stored at 4°C in the dark until analysis.
General experimental procedures
LC-MS profiling of the extracts
The LC-ESI-MS system used consisted of a Merck Hitachi® HPLC system (Hitachi Ltd., Tokyo, Japan) composed of a binary L-6200A intelligent pump, an ERC-3215α degasser (ERC Inc., Saitama, Japan), a rheodyne injector with a 20-μl loop, and a LiChroCART RP-18, endcapped (5 μm), 250 × 4 mm i.d. column (Merck, Darmstadt, Germany) coupled with a Micromass VG Quattro II mass spectrometer (Waters, Manchester, United Kingdom). The ESI-MS system was operated using Waters MassLynx 4.0 software as described in our previous work [15]. The mobile phase was a linear gradient of 100% water (containing 0.5% formic acid) to 50% acetonitrile at a flow rate of 1 mL/min over 60 min, followed by a linear gradient to 100% acetonitrile over 10 min. Mass spectrometric detection of phenolics was performed in both the positive and negative ion modes over the range of 400–1400 m/z by N2 as a nebulizer gas and a source temperature of 120°C. The chromatograms were processed by Waters MassLynx® 4.0 software (Waters Corporation, Milford, MA , USA).
Biological activities
Antitrypanosomal activity
Mature bloodstream forms of Trypanosoma brucei brucei TC221, the causative agent of Nagana (which can be used as a model for human sleeping sickness), were grown in Baltz medium supplemented with 20% inactivated fetal bovine serum and 1% penicillin-streptomycin. The cells were incubated in a humidified atmosphere containing 5% CO2 at 37°C.
Trypanocidal activity was determined by resazurin as a cell proliferation indicator dye as previously described [16]. The extracts were serially diluted with medium in a two-fold manner to attain final concentrations in the range of 250 to 3.9 μg/mL in 96-well Plates. T. b. brucei was seeded into 96-well plates at a density of 1 × 104 cells/well in 100 μL of medium. The cells were then incubated with various concentrations (3.9–250 μg/ml) of the test samples for 24 h. Ten microliters of resazurin was added to each well and incubated for a further 24 h. The absorbance of the wells was recorded by a Tecan® plate reader (Männedorf, Switzerland) at dual wavelengths of 492 and 595 nm, and the IC50 was calculated [17].
Each sample concentration was tested in triplicate, and the experiments were repeated independently twice. The maximum concentration of the solvent (DMSO) did not exceed 1.25% in the medium that contained the highest concentration of each test sample. Diminazene aceturate was used as a positive control (IC50: 0.088 μg/mL) [18].
Antimicrobial activity
Microbial strains
The antimicrobial activity was evaluated by standard strains. The Gram-positive bacteria examined included Bacillus subtilis (ATCC 6051), Staphylococcus aureus (ATCC 29213), Staphylococcus epidermidis (ATCC 14990), Streptococcus pyogenes (ATCC 12344), Streptococcus agalactiae (ATCC 27956), and Methicillin-Resistant Staphylococcus aureus (MRSA; NTCC 10442). The Gram-negative bacteria Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853) were also included, as well as the fungi Candida albicans (ATCC 90028) and Candida glabrata (ATCC MYA 2950). Multi-resistant clinical isolates from patients, such as MRSA 818014 and MRSA 818081, were also examined. All microorganisms were supplied by Medical Microbiology Laboratory, Hygiene Institute, Heidelberg University.
Inocula preparation
Prior to the test, bacterial and fungal cultures were prepared as follows. The bacterial cultures were subcultured in Columbia medium with 5% sheep blood (BD, Heidelberg, Germany) and incubated at 37°C for 24 h, while the fungal cultures were subcultured in CHROMagar® Candida (BD, Heidelberg, Germany) and incubated at 25°C for 48 h.
Agar diffusion method
Suspensions of microorganisms were prepared in a saline solution and adjusted with 0.5 McFarland Standard to a final concentration of approximately 1 × 106 CFU/mL as previously reported [14]. Mueller Hinton agar (Biomerieux, Marcy l’Étoile, France) was inoculated with the pathogens. Wells with a diameter of 6 mm were cut out by a Pasteur pipette and loaded with 40 μL of 3.2 mg/mL extracts. DMSO, ampicillin, vancomycin, and nystatin were used as controls. The diameters of the growth inhibition zones were measured in triplicate after incubation at 37°C for 24 h (bacteria) or 48 h (fungi).
Determination of minimum inhibitory concentration (MIC) and minimum microbicidal concentration (MMC) values
The MIC was determined by the broth microdilution method [19]. Various sample concentrations (0.05–25 μg/mL) were dissolved in 5% DMSO and placed in a 96-well plate (Greiner Bio-one, Frickenhausen, Germany). The final count of the microbial suspension in Mueller Hinton broth (Fluka, Buchs, Switzerland) and Sabouraud Dextrose broth (Oxoid, Hampshire, UK) was adjusted to approximately 5 × 105 CFU/mL for bacteria and fungi, respectively. The plates were incubated at 37°C for 24 h (bacteria) or 48 h (fungi)., The suspension (3 μL) from each incubated well was spread out on medium and incubated at 37°C for 24 h (bacteria) or 48 h (fungi) to determine the MMC. The MMC was defined as the lowest concentration of each extract that completely killed the microorganisms. Each test was performed in duplicate. The antibiotics ampicillin, vancomycin, and nystatin were used as positive controls.
Antiviral activity
Antiviral activity was determined by a plaque reduction assay [20]. Briefly, a confluent layer of Vero cells (CCL-81) was obtained by culturing the cells for 24 h in 0.5% CO2 at 37°C. The cells were inoculated, separately with herpes simplex virus type-1 (HSV-1), hepatitis A virus (HAV), or vesicular stomatitis virus (VSV) (1 × 10-1 ‒ 1 × 10-7 /ml) and incubated at 37°C for 1 h. The infected cell cultures (2 × 103 PFU) were washed and overlaid with DMEM containing three concentrations of each extract (6.25, 12.5, and 50 ìg/mL) for 1 h at room temperature. Each concentration was tested in three replicates, and the cultures were overlaid with nutrient agarose (2 × DMEM/1.8% agarose [v/v]) containing 25 mM MgCl2. After 72 h of incubation, the cells were fixed with 10% formaldehyde in phosphate-buffer solution (pH 7.3) for 1 h and stained with 0.5% crystal violet in 20% ethanol. The plaques were counted, and the percentage of viral inhibition was calculated as [1 - fVd/Vc)] × 100, where Vd and Vc are the numbers of plaques in the presence and absence of the test samples, respectively. Acyclovir was employed as a positive control.
Cytotoxicity
Cell cultures
Human MCF-7 (breast cancer), PANC-1 (pancreatic carcinoma), MIA PaCa-2 (pancreatic cancer), Caco-2 (colon cancer), and African green monkey kidney COS-7 (fibroblast-like epithelium) and CCL-81 (Vero) cell lines were maintained in DMEM complete medium (L-glutamine supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 U/mL streptomycin), with addition of 1 mM sodium pyruvate and 10 mM non-essential amino acids for culture of Caco-2 cells. Human CCRF-CEM (leukemia) and HL-60 (myeloid) cells were maintained in RPMI complete medium. The cells were maintained at 37°C in a humidified atmosphere containing 5% CO2. All experiments were performed with cells in the logarithmic growth phase.
Cytotoxicity and cell proliferation assay
Cytotoxicity was determined in triplicate using the MTT cell viability assay [21]. Exponentially growing cells of each cell line were seeded in 96-well plates (Greiner Labortechnik®, Frickenhausen, Germany) at 2 × 104 cells/well. The cells were cultured for 24 h, and then incubated with various concentrations of the extracts ranging from 1 to 1000 μg/mL at 37°C for 24 h. Subsequently, the cells were incubated with 0.5 mg/mL MTT for 4 h. The formed formazan crystals were dissolved in 200 μL of DMSO. The absorbance was detected at 570 nm by a Tecan® Safire II Reader (Männedorf, Switzerland). The cell viability (percentage) of three independent experiments was calculated as follows:
The cytotoxic agent doxorubicin was used as a positive control.
Apoptosis assay
The Caspase-Glo™ 3/7 Assay (Promega®, Mannheim, Germany) was used to detect caspase 3/7 activities in MIA PaCa-2 cells after treatment with each type of extract (62.5 to 1000 μg/mL). This test provides a proluminescent caspase 3/7 substrate, which contains the caspase 3-specific tetrapeptide sequence DEVD in a reagent mix, and is optimized for cell lysis and determination of caspase activities. Cells cultured in DMEM were seeded in 96-well plates and treated with the extracts (62.5 μg to 1 mg). Subsequently, 100 μL of caspase 3/7 reagent was added to the wells after 6 or 24 h of treatment, mixed, and incubated at room temperature for 30 min [22]. The luminescence was measured by a Mithras LB 940 instrument (Berthold Technologies, Bad Wildbad, Germany). Cellular apoptosis was expressed as the fold change relative to the untreated control in three independent experiments.
Data analysis
All biological experiments were repeated at least three times. Data were presented as the mean ± standard deviation (SD). The IC50 was determined as the drug concentration that resulted in a 50% reduction in cell viability or inhibition of biological activity. The IC50 values were calculated by a four parameter logistic curve (SigmaPlot® 11.0, Systat Software Inc., San Jose CA, USA). The significance of differences in data between the groups was determined by one-way analysis of variance followed by the Tukey test for equality of variances using SPSS® 11.0 (SPSS Inc., Chicago, USA). Differences were considered statistically significant at P < 0.05.
Results and discussion
HPLC profiling and chemical compositions of the extracts
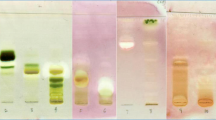
The chromatographic profiles were established by HPLC/MS (Figure 1). The ME extract (Figure 1A) revealed the presence of high contents of flavonoids, and to lesser extents lignans, sterols, and triterpenoid saponins, as previously reported by the authors [11]. The DCM extract (Figure 1B) showed a similar pattern of secondary metabolites with higher yields of lignans and terpenoids compared with those detected in the ME extract. These results are in accordance with our previously detected terpenoids in the n-hexane extract [14].
In the B. marginatum ME and DCM extracts, the following compounds (Figure 2) were identified unequivocally: quercetin and its glycosides rutin and isoquercetrin; isorhamnetin and its glycoside narcissin; (3,4-dimethoxybenzyl)-2-(3,4-methylenedioxybenzyl) butyrolactone; and marginatoxin. In addition, sterols such as β-sitosterol and α-spinasterol and their glucosides, daucosterol, stigmasterol, 7-stigmasten-3-ol, 7-stigmast-7,25-dien-3-ol, and hexadecanol were either isolated or identified from the different extracts.
Activity against T. b. brucei
The trypanocidal activities of both extracts were illustrated in Figure 3. The ME and DCM extracts showed moderate activities with IC50 values of 104.80 ± 5.11 (P = 0.0027) and 36.21 ± 2.68 (P = 0.0014) μg/mL, respectively. The selectivity indexes of the two extracts were calculated as 2.3 and 2.9, respectively, indicating low selectivity against trypanosomes compared with their cytotoxicity toward HL-60 cells.
Based on previous work [23, 24], many flavonoid aglycones exhibitantitrypanosomal activity in both in vitro and in vivo models. However, the activity of most flavonoids against T. b. brucei and the selectivity of these compounds were related to the presence of a free phenolic OH group in position 6 which can dissociate to a phenolate ion under physiological conditions [23]. Thus, it is probable that the activity of the extracts could be related to the presence of several flavonoids that could interact together in synergistic or even additive manners [25].
To our knowledge, few studies have addressed the antitrypanosomal activity of lignans [26]. However, two closely structurally related lignans, justicidin B and piscatorin, which were isolated from the aerial parts of Phyllanthus piscatorum Kunth (Euphorbiaceae), exhibited strong activity against the bloodstream forms of Trypanosoma brucei rhodesiense with IC50 values of 0.55 and 6.1 μM, respectively [27].
Antimicrobial activity
The antimicrobial activity of the ME and DCM extracts was assessed by the agar diffusion and microdilution methods, and the MIC and MMC values were determined (Tables 1 and 2). In general, both extracts showed growth inhibition of standard Gram-positive and Gram-negative bacteria in the diffusion assay. However, no inhibition zones could be detected for the two fungi and the resistant pathogenic isolates.
All of the tested Gram-positive bacteria were susceptible to the ME and DCM extracts with MIC values ranging from 0.25 to 4 mg/mL. S. agalactiae and B. subtilis were sensitive to both extracts with similar MIC values of 0.5 mg/mL. S. pyogenes was the most susceptible to the DCM extract with an MIC value of 0.25 mg/mL. The Gram-negative pathogens and fungi were less sensitive, and their MIC values were ≥ 2 mg/mL. The two extracts did not differ significantly in their antimicrobial actions for all of the susceptible microorganisms. However, they were significantly different from both ampicillin and vancomycin as seen from the difference in inhibition zones in Table 1.
Although it was expected that the ME extract of B. marginatum would possess superior activity compared with the DCM extract based on the richness and diversity of secondary metabolites, our findings did not reveal a significant difference. The expected higher activity of the ME extract was related to the presence of saikosaponins, which function as detergents and mostly exist in their mono-desmosidic forms [28]. These compounds can intercalate via their lipophilic moiety with the lipophilic membrane bilayer of the pathogens after forming a complex with cholesterol, while the hydrophilic sugar part remains outside of the cell and interacts with glycoproteins or glycolipids [28]. A loss of the membrane integrity allows other highly polar flavonoid glycosides to penetrate the pathogen and exert their effects. On this basis, the antimicrobial activity is likely to be influenced by the synergistic and additive effects of all components [28].
Antiviral activity
The ME and DCM extracts showed promising antiviral activity at a dose of 50 μg/mL. HAV was the most sensitive, showing 76.6% and 81.4% inhibition of viral replication with the ME and DCM extracts, respectively (Table 3). The antiviral activity of the different Bupleurum species have been related to the contents of saikosaponins, which exert powerful and selective antiviral activity [29–31]. However, the DCM extract with a low steroid content showed a higher activity than the ME extract against the three viruses, which might be attributed to its high contents of lignans and flavonoid aglycones.
Several modes of cytotoxic and antiviral activity were associated with lignans, including tubulin binding, reverse transcriptase, and topoisomerase inhibition [32, 33]. However, the most relevant mechanism associated with lignans involved binding to tubulin, disruption of the cellular cytoskeleton and spindle apparatus of the infected host cells, and interfering with some critical viral processes [32]. Marginatoxin, isolated from a B. marginatum extract, showed high structural similarity with podophyllotoxin, which was previously reported to show potent activity against HSV-1, measles virus, VSV, and many other viruses [34–36].
In addition, flavonoids constitute the largest group of secondary metabolites with antiviral activity in the entire plant kingdom. The several phenolic groups in the flavonoid skeleton, which can dissociate into phenolate ions and form hydrogen and ionic bonds with amino acid residues of proteins, facilitates the antiviral activity by inhibiting viral polymerase activity and binding with viral nucleic acid or viral capsid proteins, leading to a reduction in viral replication [37, 38]. Most flavonoids, including quercetin and rutin, show promising activity against HSV-I, HIV-1, HIV-2, poliovirus type 1, parainfluenza virus type 3, and respiratory syncytial virus [39–41].
Cytotoxicity and apoptosis
The cytotoxicity of the ME and DCM extracts was evaluated by eight different human cancer cell lines, Caco-2, CCL-81, CCRF-CEM, COS-7, HL-60, MCF-7, MIA PaCa-2, and PANC-1, after 24 h of incubation. The IC50 values are shown in Table 4. The cytotoxicity of the ME extract ranged from 22.5 to 576.0 μg/mL, while the DCM extract was more cytotoxic and had IC50 values ranging from 12.5 to 72.8 μg/mL. Both CCRF-CEM and MIA PaCa-2 cells were highly sensitive to both extracts, while the green monkey cells were the most resistant cell lines. Caspase 3/7 activity was used to measure the ability of the extracts to initiate the apoptotic cascade. Both extracts activated caspase 3/7. The increase was most apparent with the DCM extract, for which a 1 mg/mL dose produced 19-fold stimulation (Figure 4). The tested extracts showed higher selectivity for cancer cells when compared with normal hepatocytes. The ME extract had no cytotoxic activity against normal human cells over the range of 1–1000 mg/mL (unpublished data).
Several other Bupleurum species showed similar cytotoxicity against different cell lines [42–44]. The mechanism of this cytotoxicity was attributed to the saikosaponin content, especially the saponins with an epoxy bridge [42]. Cytotoxicity is mainly mediated through cell cycle arrest in the G2/M phase or inhibition of tubulin polymerization [45]. In addition, induction of apoptosis via the activation of kinases 1/2 and several caspases might be involved [43–45].
Furthermore, quercetin and lignans contributed to the overall cytotoxicity of both extracts. Quercetin blocks signal transduction pathways by inhibiting many kinases, including protein tyrosine kinases and serine/threonine protein kinases, resulting in arrest of the cell cycle at the late G1 phase [46] with a proapoptotic activity through activation of KRAS and PI3K [47]. Most lignans, especially those with a structural similarity to podophyllotoxin are known cytotoxic agents [48]. These agents bind to tubulin, leading to inhibition of microtubule formation, and inhibit DNA topoisomerase, thus blocking cell division in the late G2[49].
The low cytotoxicity of both extracts toward Caco-2 cells compared with the other tested cancer cell lines may arise through the overexpression of multidrug-resistance genes, such as MDR1 and MRP1, in this cell line [50, 51]. These ATP-dependent efflux transporters facilitated the efflux of xenobiotics from Caco-2 cells, thereby reducing th\e internal concentration of cytotoxic drugs and thus their activity [52].
In this work, we investigated the antimicrobial activity of Bupleurum extracts, especially against Gram-positive bacteria such as Streptococcus and Bacillus. Moreover, the antitrypanosomal and antiviral activities of the DCM extract were explored against three different viruses. Further studies of the isolated compounds present in the extracts, particularly lignans, which might serve as lead compounds and could be modified to increase their selectivity were required. However, clinical reports should be collected from patients using these Bupleurum preparations to monitor the efficacy, as well as any side effects or herbal drug interactions.
Conclusions
In this study, ME and DCM extracts of B. marginatum showed anti-infective and antiproliferative effects.
References
Cordell GA: Natural products in drug discovery – Creating a new vision. Phytochem Rev. 2002, 1: 261-273. 10.1023/A:1026094701495.
Yaniv Z, Bachrach U: Handbook of medicinal plants. 2005, New York: Food Products Press : Haworth Medical Press
Mabberley DJ: Mabberley's plant-book: a portable dictionary of plants, their classification and uses. 3rd edn. Cambridge, UK. 2008, New York: Cambridge University Press
Pan S-L: Bupleurum species: scientific evaluation and clinical applications. 2006, Boca Raton: CRC/Taylor & Francis
World Health Organization: WHO Monographs on Selected Medicinal Plants Vol 1. 1999, Geneva: World Health Organization
Ashour ML, Wink M: Genus Bupleurum: a review of its phytochemistry, pharmacology and modes of action. J Pharm Pharmacol. 2011, 63: 305-321. 10.1111/j.2042-7158.2010.01170.x.
Wu J-N: An illustrated Chinese materia medica. 2005, New York: Oxford University Press
Fundukian LJ: The Gale encyclopedia of alternative medicine. 2009, Gale, Cengage Learning: Detroit, 3
Zhou J: Encyclopedia of traditional Chinese medicines vol 1 - molecular structures, pharmacological activities, natural sources and applications. 2011, New York: Springer
Huang HQ, Zhang X, Lin M, Shen YH, Yan SK, Zhang WD: Characterization and identification of saikosaponins in crude extracts from three Bupleurum species using LC-ESI-MS. J Sep Sci. 2008, 31: 3190-3201. 10.1002/jssc.200800120.
Ashour ML, El-Readi MZ, Tahrani A, Eid SY, Wink M: A novel cytotoxic aryltetraline lactone from Bupleurum marginatum (Apiaceae). Phytochem Lett. 2012, 5: 387-392. 10.1016/j.phytol.2012.03.009.
Zhitao L, Minjian Q, Zhengtao W: Study on the constituent s of the roots of Bupleurum Marginatum. J China Pharmaceut Uni. 2003, 34: 305-308. [In Chinese]
Liu Y, Zhang T-T, Zhou J-S, Wang Q: Three new arylnaphthalide lignans from the aerial parts of Bupleurum marginatum WALL. ex DC. Helv Chim Acta. 2008, 91: 2316-2320. 10.1002/hlca.200890252.
Ashour ML, El-Readi M, Youns M, Mulyaningsih S, Sporer F, Efferth T, Wink M: Chemical composition and biological activity of the essential oil obtained from Bupleurum marginatum (Apiaceae). J Pharm Pharmacol. 2009, 61: 1079-1087.
Hamdan D, El-Readi MZ, Tahrani A, Herrmann F, Kaufmann D, Farrag N, El-Shazly A, Wink M: Chemical composition and biological activity of Citrus jambhiri Lush. Food Chem. 2011, 127: 394-403. 10.1016/j.foodchem.2010.12.129.
Nibret E, Ashour ML, Rubanza CD, Wink M: Screening of some Tanzanian medicinal plants for their trypanocidal and cytotoxic activities. Phytother Res. 2010, 24: 945-947.
Huber W, Koella JC: A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 1993, 55: 257-261. 10.1016/0001-706X(93)90083-N.
Nibret E, Sporer F, Asres K, Wink M: Antitrypanosomal and cytotoxic activities of pyrrolizidine alkaloid-producing plants of Ethiopia. J Pharm Pharmacol. 2009, 61: 801-808. 10.1211/jpp.61.06.0014.
Mulyaningsih S, Sporer F, Reichling J, Wink M: Antibacterial activity of essential oils from Eucalyptus and of selected components against multidrug-resistant bacterial pathogens. Pharm Biol. 2011, 49: 893-899. 10.3109/13880209.2011.553625.
Abou-Karam M, Shier WT: A simplified plaque reduction assay for antiviral agents from plants. Demonstration of frequent occurrence of antiviral activity in higher plants. J Nat Prod. 1990, 53: 340-344. 10.1021/np50068a011.
Mosmann T: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983, 65: 55-63. 10.1016/0022-1759(83)90303-4.
Reimer TA, Anagnostopoulos I, Erdmann B, Lehmann I, Stein H, Daniel P, Dorken B, Rehm A: Reevaluation of the 22-1-1 antibody and its putative antigen, EBAG9/RCAS1, as a tumor marker. BMC Cancer. 2005, 5: 47-10.1186/1471-2407-5-47.
Tasdemir D, Kaiser M, Brun R, Yardley V, Schmidt TJ, Tosun F, Ruedi P: Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: in vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob Agents Chemother. 2006, 50: 1352-1364. 10.1128/AAC.50.4.1352-1364.2006.
Wink M: Medicinal plants: source of anti-parasitic secondary metabolites. Molecules. 2012, 17: 12771-12791. 10.3390/molecules171112771.
Mamadalieva NZ, Herrmann F, El-Readi MZ, Tahrani A, Hamoud R, Egamberdieva DR, Azimova SS, Wink M: Flavonoids in Scutellaria immaculata and S. ramosissima (Lamiaceae) and their biological activity. J Pharm Pharmacol. 2011, 63: 1346-1357. 10.1111/j.2042-7158.2011.01336.x.
Hoet S, Opperdoes F, Brun R, Quetin-Leclercq J: Natural products active against African trypanosomes: a step towards new drugs. Nat Prod Rep. 2004, 21: 353-364. 10.1039/b311021b.
Gertsch J, Tobler RT, Brun R, Sticher O, Heilmann J: Antifungal, antiprotozoal, cytotoxic and piscicidal properties of Justicidin B and a new arylnaphthalide lignan from Phyllanthus piscatorum. Planta Med. 2003, 69: 420-424.
Wink M: Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr Drug Metab. 2008, 9: 996-1009. 10.2174/138920008786927794.
Ushio Y, Abe H: Inactivation of measles virus and herpes simplex virus by saikosaponin d. Planta Med. 1992, 58: 171-173. 10.1055/s-2006-961422.
Chiang LC, Ng LT, Liu LT, Shieh DE, Lin CC: Cytotoxicity and anti-hepatitis B virus activities of saikosaponins from Bupleurum species. Planta Med. 2003, 69: 705-709.
Cheng PW, Chiang LC, Yen MH, Lin CC: Bupleurum kaoi inhibits Coxsackie B virus type 1 infection of CCFS-1 cells by induction of type I interferons expression. Food Chem Toxicol. 2007, 45: 24-31. 10.1016/j.fct.2006.06.007.
Charlton JL: Antiviral activity of lignans. J Nat Prod. 1998, 61: 1447-1451. 10.1021/np980136z.
Wink M, Van Wyk B-E: Mind-altering and poisonous plants of the world. 2008, Portland: Timber Press
Bedows E, Hatfield GM: An investigation of the antiviral activity of Podophyllum peltatum. J Nat Prod. 1982, 45: 725-729. 10.1021/np50024a015.
MacRae WD, Hudson JB, Towers GH: The antiviral action of lignans. Planta Med. 1989, 55: 531-535. 10.1055/s-2006-962087.
Jassim SA, Naji MA: Novel antiviral agents: a medicinal plant perspective. Appl Microbiol. 2003, 95: 412-427. 10.1046/j.1365-2672.2003.02026.x.
Cushnie TP, Lamb AJ: Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005, 26: 343-356. 10.1016/j.ijantimicag.2005.09.002.
Naithani R, Huma LC, Holland LE, Shukla D, McCormick DL, Mehta RG, Moriarty RM: Antiviral activity of phytochemicals: a comprehensive review. Mini-Rev Med Chem. 2008, 8: 1106-1133. 10.2174/138955708785909943.
Kaul TN, Middleton E, Ogra PL: Antiviral effect of flavonoids on human viruses. J Med Virol. 1985, 15: 71-79. 10.1002/jmv.1890150110.
Ohnishi E, Bannai H: Quercetin potentiates TNF-induced antiviral activity. Antiviral Res. 1993, 22: 327-331. 10.1016/0166-3542(93)90041-G.
Kim HJ, Woo ER, Shin CG, Park H: A new flavonol glycoside gallate ester from Acer okamotoanum and its inhibitory activity against human immunodeficiency virus-1 (HIV-1) integrase. J Nat Prod. 1998, 61: 145-148. 10.1021/np970171q.
Hsu YL, Kuo PL, Weng TC, Yen MH, Chiang LC, Lin CC: The antiproliferative activity of saponin-enriched fraction from Bupleurum Kaoi is through Fas-dependent apoptotic pathway in human non-small cell lung cancer A549 cells. Biol Pharm Bull. 2004, 27: 1112-1115. 10.1248/bpb.27.1112.
Cheng YL, Lee SC, Lin SZ, Chang WL, Chen YL, Tsai NM, Liu YC, Tzao C, Yu DS, Harn HJ: Anti-proliferative activity of Bupleurum scrozonerifolium in A549 human lung cancer cells in vitro and in vivo. Cancer Lett. 2005, 222: 183-193. 10.1016/j.canlet.2004.10.015.
Fujioka T, Yoshida K, Shibao H, Nagao T, Yoshida M, Matsunaga K, Takata J, Karube Y, Iwase Y, Okabe H, Mihashi K: Antiproliferative constituents from umbelliferae plants. IX. New triterpenoid glycosides from the fruits of Bupleurum rotundifolium. Chem Pharm Bull. 2006, 54: 1694-1704. 10.1248/cpb.54.1694.
Chen YL, Lin SZ, Chang WL, Cheng YL, Harn HJ: Requirement for ERK activation in acetone extract identified from Bupleurum scorzonerifolium induced A549 tumor cell apoptosis and keratin 8 phosphorylation. Life Sci. 2005, 76: 2409-2420. 10.1016/j.lfs.2004.09.044.
Csokay B, Prajda N, Weber G, Olah E: Molecular mechanisms in the antiproliferative action of quercetin. Life Sci. 1997, 60: 2157-2163. 10.1016/S0024-3205(97)00230-0.
Xavier CP, Lima CF, Preto A, Seruca R, Fernandes-Ferreira M, Pereira-Wilson C: Luteolin, quercetin and ursolic acid are potent inhibitors of proliferation and inducers of apoptosis in both KRAS and BRAF mutated human colorectal cancer cells. Cancer Lett. 2009, 281: 162-170. 10.1016/j.canlet.2009.02.041.
MacRae WD, Towers GHN: Biological activities of lignans. Phytochemistry. 1984, 23: 1207-1220. 10.1016/S0031-9422(00)80428-8.
Wink M: Plant secondary metabolism: diversity, function and its evolution. Nat Prod Commun. 2008, 3: 1205-1216.
Seithel A, Karlsson J, Hilgendorf C, Bjorquist A, Ungell AL: Variability in mRNA expression of ABC- and SLC-transporters in human intestinal cells: comparison between human segments and Caco-2 cells. Eur J Pharm Sci. 2006, 28: 291-299. 10.1016/j.ejps.2006.03.003.
Wink M, Ashour ML, El-Readi MZ: Secondary metabolites from plants inhibiting ABC transporters and reversing resistance of cancer cells and microbes to cytotoxic and antimicrobial agents. Front Microbiol. 2012, 3: 130-
El-Readi MZ, Hamdan D, Farrag N, El-Shazly A, Wink M: Inhibition of P-glycoprotein activity by limonin and other secondary metabolites from Citrus species in human colon and leukaemia cell lines. Eur J Pharmacol. 2010, 626: 139-145. 10.1016/j.ejphar.2009.09.040.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MLA and MW designed the study. MLA, MZL, RH, SYE, SHE, EN, FH, MY, AT and DKperformed the experiments. MLA and MW wrote the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ashour, M.L., El-Readi, M.Z., Hamoud, R. et al. Anti-infective and cytotoxic properties of Bupleurum marginatum. Chin Med 9, 4 (2014). https://doi.org/10.1186/1749-8546-9-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1749-8546-9-4