Abstract
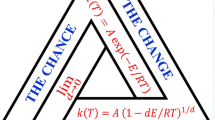
By the temporal Uncertainty principle, chemical systems may be described in terms of ‘kinetic’ or ‘thermodynamic’ complementary formulations, based on rate or equilibrium constants, and free energy changes respectively. Thereby, the paradox of the dichotomous formulations of the Curtin-Hammett principle is resolved. A new formulation of the principle is suggested, and a fundamental conflict with transition state theory is indicated.
Similar content being viewed by others
References
P. Laslo and P. Stang, Organic Spectroscopy: Principles and Applications, Harper and Row, New York, 1971, pp. 70, 147.
H. Primas, Chimia, 36, 293 (1982).
L.P. Hammett, Physical Organic Chemistry, 2nd. Ed. McGraw-Hill, New York, 1970, pp. 117–120; (b) J.I. Seeman, Chem. Rev., 83, 83 (1983); (c) W.G. Dauben and K.S. Pitzer, in: M.S. Newman (Ed.), Steric Effects in Organic Chemistry, John Wiley, New York, 1956, pp. 44–47; (d) N.S. Zefirov, Tetrahedron, 33, 2719 (1977); (e) J.I. Seeman and W.A. Farone, J. Org. Chem., 43, 1854 (1978); (f) E.L. Eliel, Stereochemistry of Carbon Compounds, McGraw-Hill, New York, 1962, pp. 151–152; (g) E.L. Eliel, S.H. Wilen and L.N. Mander, Stereochemistry of Organic Compounds, John Wiley, New York, 1994, pp. 647–655; (h) IUPAC Physical Organic Glossary, Pure Applied Chem., 66, 1077 (1994); (i) IUPAC Pysical Organic Glossary, Pure Applied Chem., 55, 1281 (1830).
S. Chandrasekhar, Res. Chem. Intermed. 22, 137 (1996).
K.J. Laidler, Theories of Chemical Reaction Rates, McGraw-Hill, New York, 1969, pp. 72–73.
L.P. Hammett, Ref. 3,, pp. 117–118; J.I. Seeman, Ref. 3, Chem. Rev., 83, (1983), p. 118; J.E. Leffler and E. Grunwald, Rates and Equilibria of Organic Reactions as Treated by Statistical. Thermodynamic and Extrathermodynamic Methods, John Wiley, New York, 1963, pp. 119–120.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chandrasekhar, S. Complementarity and the temporal Uncertainty principle. Insight into the dichotomous formulations of the Curtin-Hammett principle. A new formulation. Res. Chem. Intermed. 23, 55–62 (1997). https://doi.org/10.1163/156856797X00394
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856797X00394