Abstract
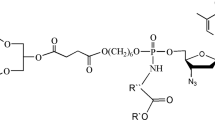
One of the approaches to enhance the bioavailability of nucleoside reverse transcriptase HIV inhibitors is the design of their prodrugs based on 1,3-diacylglycerols, which may simulate metabolic pathways of natural lipids, thus supporting the efficacy of drug delivery to the target cells. Glycerolipid AZT conjugates with different functional phosphoric centers were synthesized by the H-phosphonate technique. Stability of the prepared prodrugs against chemical and enzymatic hydrolysis (in buffer solutions and in the presence of pancreatic lipase), as well as their anti-HIV activity against the HIV-1899A strain in human Tlymphoid MT-4 cells, were studied.
Similar content being viewed by others
Abbreviations
- AZT:
-
3′-azido-3′-deoxythymidine
- Cap:
-
capronoyl (hexanoyl)
- DCC:
-
N,N′-dicyclohexylcarbodiimide
- DMAP:
-
4-dimethylaminopyridine
- Gro:
-
glycerol
- Hx:
-
hexyl, Palm, palmitoyl
- PIPES:
-
piperazine-1,4-bis(2-ethanesulfonic acid)
- Piv:
-
pivaloyl
- Py:
-
pyridine
- Suc:
-
succinyl
- Thy:
-
thymine
- HIV:
-
human immunodeficiency virus
- MT-4:
-
human T-lymphoid
- MTT:
-
the colorimetric assay for the determination of cell proliferative activity
- TCID:
-
tissue culture infectious dose
- CPE:
-
cytopathic effect
References
Broder, S., Antiviral. Res., 2010, vol. 85, pp. 1–18.
Ohrui, H., Proc. Jpn. Acad. Ser., 2011, vol. 87, no. 3, pp. 53–65.
Mehellou, Y., J. Med. Chem., 2010, vol. 53, pp. 521–538.
Clercq, E., J. Clin. Virol., 2004, vol. 30, pp. 115–133.
Cihlar, T. and Ray, S.A., Nucleoside Antiviral. Res., 2010, vol. 85, pp. 39–58.
Li, F., Maag, H., and Alfredson, T., J. Pharm. Sci., 2008, vol. 97, no. 3, pp. 1109–1134.
Ellis, R., Prog. Neurobiol., 2010, vol. 91, pp. 185–187.
Gumina, G., Choi, Y., and Chu, C.K., Recent Adv. Antivir. Nucleosides, 2003, vol. 1, pp. 1–76.
Clercq, E., Antivir. Res., 2005, vol. 67, pp. 56–75.
Wong, A. and Toth, I., Curr. Med. Chem., 2001, vol. 8, no. 9, pp. 1123–1136.
Lambert, D.M., Eur. J. Pharm. Sci., 2000, vol. 11, suppl. 2, pp. S15–S27.
Charman, W.N. and Porter, C.J.H., Adv. Drug Deliv. Rev., 1996, vol. 19, pp. 149–169.
Ali, S.M., Khan, A.R., Ahmad, M.U., Chen, P., Sheikh, S., and Ahmad, I., Bioorg. Med. Chem. Lett., 2005, vol. 15, pp. 2571–2574.
Menendez-Arias, L., Virus Res., 2008, vol. 134, pp. 124–146.
Meiera, C., Loreya, M., Clercq, E., and Balzarini, J., Bioorg. Med. Chem. Lett., 1997, vol. 7, no. 2, pp. 99–104.
Peterson, L.W. and McKenna, C.E., Expert Opin. Drug Deliv., 2009, vol. 6, no. 4, pp. 405–420.
Bentley, P.H. and McCrae, W., Org. Chem., 1970, vol. 36, no. 6, pp. 2082–2083.
Scriba, G.K.E., Arch. Pharm. (Weinheim), 1993, vol. 326, pp. 477–481.
Lonshakov, D.V., Baranova, E.O., Lyutik, A.I., Shastina, N.S., and Shvets, V.I., Khim.-Farm. Zh., 2010, vol. 44, no. 10, pp. 27–34.
Xiao, Q., Sun, J., Sun, Q.JuY., Zhao, Y., and Cui, Y., Synthesis, 2003, no. 1, pp. 107–111.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.S. Shastina, T.Yu. Maltseva, L.N. D’yakova, O.A. Lobach, M.S. Chataeva, D.N. Nosik, V.I. Shvetz, 2013, published in Bioorganicheskaya Khimiya, 2013, Vol. 39, No. 2, pp. 184–193.
Rights and permissions
About this article
Cite this article
Shastina, N.S., Maltseva, T.Y., D’yakova, L.N. et al. Synthesis, properties, and Anti-HIV activity of new lipophilic 3′-azido-3′-deoxythymidine conjugates containing functional phosphoric linkages. Russ J Bioorg Chem 39, 161–169 (2013). https://doi.org/10.1134/S1068162013020118
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162013020118